MRD:01336581
Excercise 1
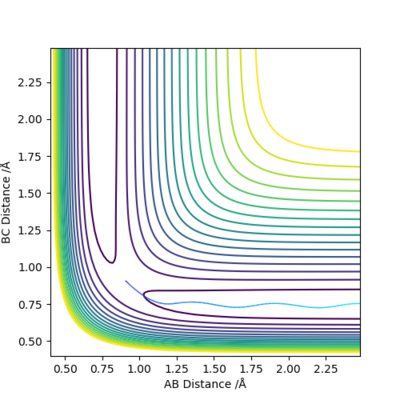
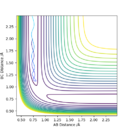
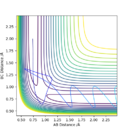
The reaction transition state happens at a saddle point on the energy surface.
and
are of opposite sign.
Good, but you need to explain what do those two maths functions mean. Why the first derivatives are zero and the second derivatives are opposite signs ? Do your r1, r2 represent the two distances AB and BC? if so, I'm afraid it is not really correct. Sw2711 (talk) 14:32, 3 June 2019 (BST)
The transition state is located between rts = 0.907742 Å and 0.907743 Å. How did you find the values? Sw2711 (talk) 14:32, 3 June 2019 (BST)
The dynamics simulation differs from the MEP simulation by showing the vibration of the product molecule.
Okay, why is it? Is it the only difference you've observed? Sw2711 (talk) 14:32, 3 June 2019 (BST)
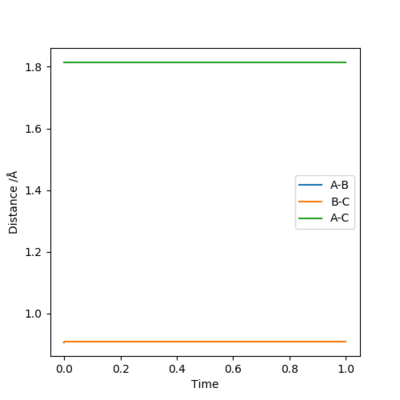
For the initial conditions r1 = 0.74 and r2 = 2.0
Transition state theory assumes that the reaction passes over the lowest energy point on the energy surface, i.e. the saddle point. This does not always happen if the reactants have higher energies. This can lead to recrossing the transition state. If the tranisiton state is recrossed, any rate calulations are unlikely to be accurate, since the time for the reaction to complete is longer than if the transition state was not recrossed. The transition state theory rate predictions form an upper bound for the rate constant.
This part is good. But you need to be aware that there are a few more assumptions in TST. Sw2711 (talk) 14:32, 3 June 2019 (BST)
Excercise 2
The potential energy surface shows that the reaction of F with H2 is exothermic, and the reaction of HF with H is endothermic.
For this simulation the F atom is in positoin A, so the left hand side is the F + H2, and the right hand side is the HF + H side. the left hand side is higher in energy, so going from F + H2 to HF + H is exothermic. This shows that the H-F bond is stronger than the H-H bond, since overall energy is released on the formation of the H-F bond over the H-H bond.
This part is good. Sw2711 (talk) 14:37, 3 June 2019 (BST)
The transition state is approximately at F-H = 1.81 Å and H-H = 0.745 Å.
You need to explain your approach.Sw2711 (talk) 14:37, 3 June 2019 (BST)
A minimum energy pathway from these values gave a value for the activation energy of the HF + H reaction of 30 kcalmol-1.
A minimum energy pathway calculated from F-H = 1.82 Å and H-H = 0.745 Å resulted in a value for the activation energy of ~0.25 kcalmol-1.
This part is goodSw2711 (talk) 14:37, 3 June 2019 (BST)
A reactive pathway has initial conditions rFH = 2 Å, rHH = 0.74 Å, pFH = -0.8, pHH = 0.
The reaction energy is released by the formation of the H-F bond. Quantum mechanically, the constructive overlap of electron wavefunctions is stabilising, which releases energy to be converted into vibrational or translational forms. This can be shown by removing the fluorine atom, leaving just the atomisation of a hydrogen molecule, which is endothermic. Okay, so, 1. how do you see the energy is conserved in your program. 2. in reality, what kind of technique you need to do, to remove the fluorine atom, according to what you said? Sw2711 (talk) 14:37, 3 June 2019 (BST)
Polanyi's rules state that vibrational energy is more useful for promoting reactions with a late transition state, and translational energy is more useful in promoting reactions with an early transition state. For the reaction between HF and H, the transition state is very late, so a small amount of energy in the vibration of the HF molecule results in a reaction, whereas a much larger amount of translational energy on the H atom results in an unreactive pathway. The inverse is true for the reaction between F and H2.
If you have tested using HF+H system, you need to show me. Sw2711 (talk) 14:39, 3 June 2019 (BST)