MRD:00940575
Molecular Reaction Dynamics Write-up
Exercise 1
On a potential energy surface diagram, how is the transition state mathematically defined? How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?
The energy as a function of the positons of atoms needs to be worked out first. The first order derivative of this gives stationary points. These points correspond to the physically stable reactant and product, and transition state. The transition state is defined by the saddle point on a potential energy surface diagram. This point has the highest energy on the reaction pathway line, so it can be distinguished easily from other stationary points.
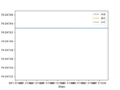
Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.
At rab and rbc = 90. both bonds has an oscillation that superimpose on each other as they have the same length. Many values of r was put in until the value converged to a flat line with no oscillation that showed that rts= 90.76 pm Good. well done. Mak214 (talk) 10:21, 7 July 2020 (BST)
Comment on how the mep and the trajectory you just calculated differ.
In the previous trajectory, rab and rbc was 90.76pm, but when rbc was chaged to 91.76 pm, it increases and rab decreases and asymptotes to a rab=74.04743. Momentum for ab asymptotes too. MEP and Dynamics are different calculations which treat the momentum of the system in completely different ways. In MEP calculations the momentum is reset to 0 at every timestep, meaning the system only responds to the gradient of the potential energy surface at each point - so it will find the nearest energy minimum. Dynamics trajectories are more realistic simulations where the system will hold on to momentum and so we see vibration etc with dynamics calculations. Mak214 (talk) 10:21, 7 July 2020 (BST)
Complete the table above by adding the total energy, whether the trajectory is reactive or unreactive, and provide a plot of the trajectory and a small description for what happens along the trajectory. What can you conclude from the table?
rbc =74 pm , rab = 200 pm
| p1/ g.mol-1.pm.fs-1 | p2/ g.mol-1.pm.fs-1 | Etot | Reactive? | Description of the dynamics | Illustration of the trajectory |
|---|---|---|---|---|---|
| -2.56 | -5.1 | -414.28 | reactive | rab and rbc has crossed once at the transition state. r ab is oscillating and rbc is increasing so it has reacted. | |
| -3.1 | -4.1 | -420.08 | unreactive | rab and rbc does not cross, as the momentum was not enough to overcome the barrier it does not react | |
| -3.1 | -5.1 | -413.97 | reactive | rab and rbc has crossed once at the transition state. r ab is oscillating and rbc is increasing so it has reacted. | |
| -5.1 | -10.1 | -357.26 | unreactive | rab and rbc has crossed twice . rab is smaller than rbc for a short while, this means p2 was too large so even the energy was enough to overcome the barrier, the transition state was not formed. | |
| -5.1 | -10.6 | -349.47 | reactive | rab and rbc has crossed three times. The saddle point where the transition state is formed is when they meet for the third time. |
Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values? The key postulate of TST which is proven to be an overestimate of the rate here is the assumption that once the transition state has been reached then the reaction will always go to the products. Your fourth entry in the table above shows that this is not always the case, and that an equilibrium may exist between the TS and the products as well as between Reactants and TS... There is also an oversight in TST which does not account for quantum tunnelling, although we could not model for this in this lab, and the overall effect would be small. I would advise you to read up on TST, maybe you could read a classmates wiki page to learn more!! Mak214 (talk) 10:21, 7 July 2020 (BST)
The transition state theory uses the surface that intersects with the saddle point. This overestimates the rate of reaction.
It is such a shame that you didnt have time to finish this lab, there are some important concepts that were introduced, please take some time to read through the literature linked to the lab so you don't miss out!! Overall there is not a lot of time that has gone into this report and you have not engaged with the theory to fully answer the questions. See my comments above. Mak214 (talk) 10:21, 7 July 2020 (BST)