MRD01506162
3/5 - A good report overall. There are several gaps in your knowledge that you should fill before moving on (see comments). I would have liked to have seen more detail in places (highlighted throughout). Good use of figures!
Exercise 1: H + H2 system
Dynamics from the transition state region
1. The transition state is mathematically defined as having ∂V(r1)/∂r1 = ∂V(r2)/∂r2=0 and ∂V2(ri)/∂ri <0 This describes a potential energy maximum, rather than a TS. What mathematical structure does a TS resemble and how would you define it mathematically? The energy goes down most deeply at transition state along the mininum energy path linking reactants and products. A transition state can be identified by having constant internuclear distances over time.A local minimum has ∂V2(ri)/∂ri >0.
Trajectories from r1 =r2: locating the transition state


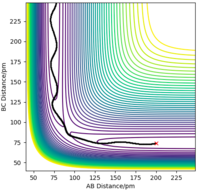
2. My best estimate of the transition state position is r1 = r2= 90.775 pm. The trajectory with this estimate and zero initial momentum has constant internuclear distances throughout the time. It doesn't "roll" towards the reactants or products proving that the trajectory is exactly at transition state.
Correct TS estimate, well done
Trajectories from r1 = rts + δ, r2 = rts
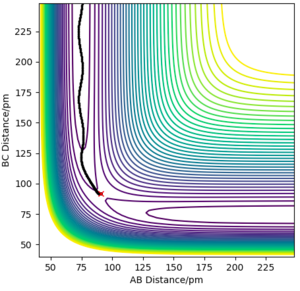
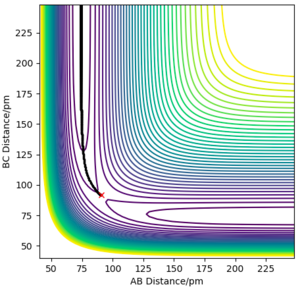
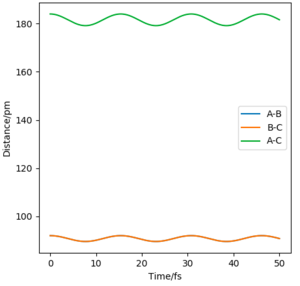
3. As shown in the pictures above, Mep doesn't have oscillatory behaviour corresponding to vibrations since momenta are always reset to zero in each time step. Meanwhile the trajectory calculated from calculation type being Dynamics has oscillations .
Good. A little more detail would be useful. How is the direction od descent in the PES calculation determined?
Reactive and unreactive trajectories
4.
One can conclude from the table that in order to have a reactive trajectory, translational energy of incoming atom needs to pair up with adequate vibrational energy of the molecule e.g. high, high and low, low.
I'm not quite sure what you're getting at here. In general, it is hard to predict which barriers will be recrossed simply from looking at trajectories.
Transition State Theory

5. Transition State Theory relies on three key assumptions and they have different effects in causing the differences between the estimated reaction rates and the experimental values. In this case, we are using the simulation as a much simpler model of real-life experiments. The first assumption which states all trajectories with KE>Ea are reactive overestimates the reaction rates since in reality barrier recrossing could have occurred. Above is an illustration of barrier recrossing. The second assumption which states the transition states are in quasi equilibrium with the reactants varies the estimated value from the real one to a limited extent. The third assumption which states quantum tunneling is not to be considered underestimates the reaction rate since in reality quantum tunneling does occur. Unfortunately, no illustration from the simulation can be done since our simulation doesn't take quantum tunneling into account either. Overall, Transition State Theory overestimates reaction rates since there are much more barrier recrossing than quantum tunneling in real life experiments.
Good!
Exercise 2: F-H-H system
PES Inspection
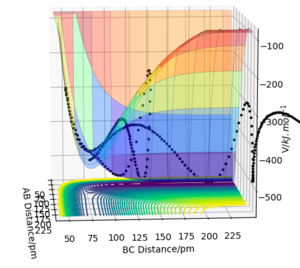
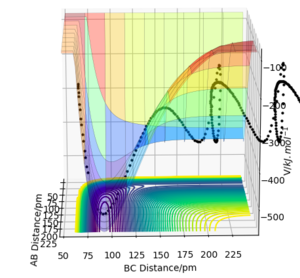
6. By inspecting the surface plots of reactive trajectories, F + H2 is exothermic and H + HF is endothermic. This is because H-F has higher bond enthalpy which means it is stronger than H-H. In F+ H2, more energy is released to form H-F than required to break H-H. In H + HF, more energy is required to break H-F than released to form H-H.
Good!
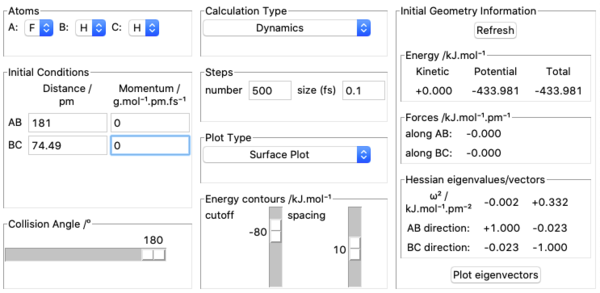



7. Transition state was located by using Hammond postulate. I understand that since F + H2 is exothermic, it has an early transition state and the transition state is reactant-like. Therefore I started by using A=F, B=H, C=H, initial conditions set as rAB= 200 pm, rBC= 74 pm, pAB= 0 g.mol-1.pm.fs-1, pBC= 0 g.mol-1.pm.fs-1 and varied AB and BC distances in order to make forces along AB and BC equal to zero. The approximate position of transition state found is rHF=181 pm, rHH=74.49 pm which has zero force along the bonds as shown in the screenshot above. Three internuclear distances vs time plots are also shown above to further demonstrate the validity of the position of transition state.
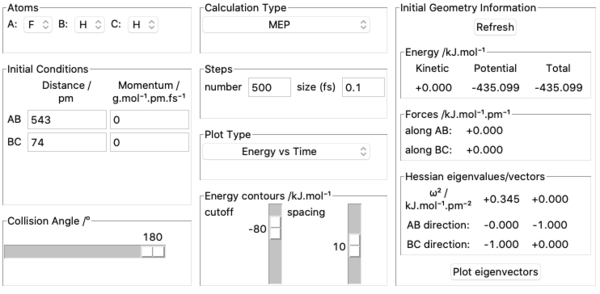

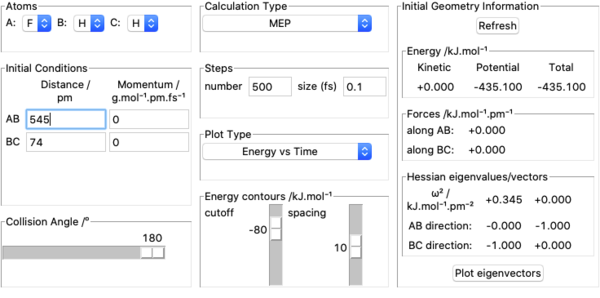

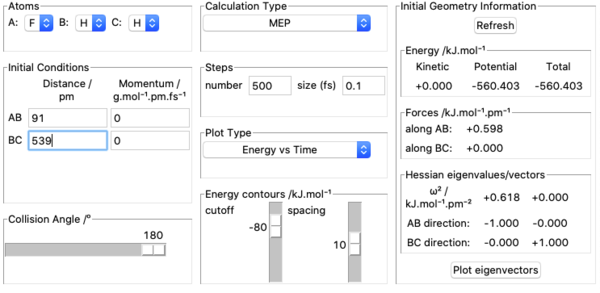

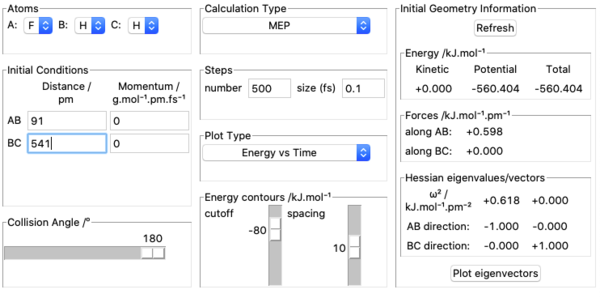
8. Activation energy of F + H2 was found by measuring potential energies of reactants, transition state and products with kinetic energy=0, momenta=0 and calculation type being MEP. Potential energy of reactants was found by placing the incoming F atom very far away from H2 molecule at equilibrium bond length =74 pm and kept increasing the distance between F and H2 until potential energy stopped changing. This resulted in F having no effect on H2 's potential energy which was found out to be -435.100 kJ mol-1. Above pictures are an illustration of the approach. The potential energy of transition state was recorded by setting initial conditions in transition state position approximated above and it was found out to be -433.981 kJ mol-1. Potential energy of the products was found by using the same method to find the potential energy of reactants and was found out to be -560.404 kJ mol-1. Above pictures are illustrations of the process.H + HF is a reverse reaction of F + H2 and its activation energy can be calculated from these values as well. Ea for F + H2 was therefore found out to be -433.981-(-435.100)=1.119 kJ mol-1. Ea for H + HF was found out to be -433.981-(-560.404)=126.423 kJ mol-1.
Good estimates for Ea in both directions, well done.
Reaction dynamics
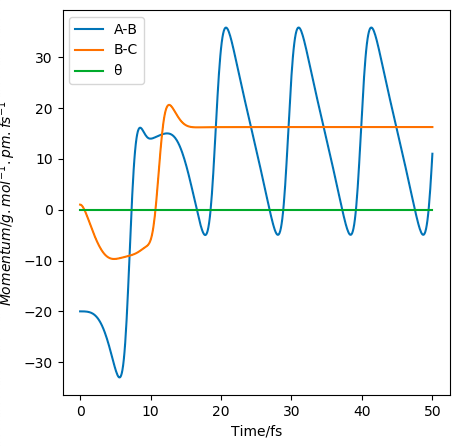
9. The potential energy released in the reaction of F + H2 is first converted to kinetic energy which is used to promote the products in vibrational ground state to vibrational first state. The vibrational activation would cause an increase in overtone intensity and decrease in fundamental intensity in IR absorbance spectrum. Overtime, the vibrational first state relaxes back to the ground state emitting IR. The vibrational relaxation would cause overtone intensity to decrease and fundamental peak intensity to increase in IR absorbance spectrum. The mechanism of the release of the reaction energy can be thus experimentally confirmed by measuring the emission of IR during vibrational relaxation by FTIR Not FTIR, as it doesn't have adequate time resolution, but yes, you're largely correct..


10. H + HF is an endothermic reaction thus has a late transition state which according to Hammond's postulate, adopts the product structure. As shown above are the surface plots for H + HF with H having high translational energy, HF having low vibrational energy and H having low translational energy, HF having high vibrational energy respectively. In the case of high translational energy of incoming H atom and high vibrational energy of HF molecule, the incoming H atom flings down the reaction channel, hits the potential wall with high translational energy and therefore bounces back. This leads to an unreactive trajectory. In the case of low translational energy of incoming H atom and high vibrational energy of HF molecule, incoming H atom approaches HF molecule whose direction of vibrational motion aligns with the direction that goes to the products. Since H has low energy, it doesn't hit the wall and bounce back. Also, HF's vibrational motion is with sufficient amplitude to cross the energy barrier and form products. This results in a reactive trajectory. According to Polanyi's empirical rules, vibrational energy is more efficient in promoting a reaction with late transition state meanwhile translational energy is more efficient in promoting a reaction with early transition state.
Good examples. Did you take any examples for the F+H2 reaction?