MD5317
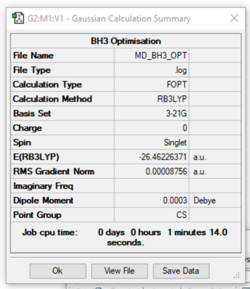
BH3
B3LYP/3-21G level, BH3
Item Value Threshold Converged? Maximum Force 0.000217 0.000450 YES RMS Force 0.000105 0.000300 YES Maximum Displacement 0.000919 0.001800 YES RMS Displacement 0.000441 0.001200 YES Predicted change in Energy=-1.635268D-07 Optimization completed. -- Stationary point found.
https://wiki.ch.ic.ac.uk/wiki/images/1/1a/MD_BH3_OPT.LOG
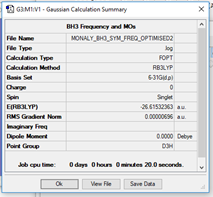
B3LYP/6-31G(d,p) level
Frequency Analysis6-31G(d,p) BH3
| Low frequencies | -7.9073 | -1.6385 | -0.0054 | 0.6256 | 6.5697 | 6.7709 |
| 1162.9662 | 1213.1623 | 1213.1650 |
Item Table for Frequency
Item Value Threshold Converged? Maximum Force 0.000014 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000055 0.001800 YES RMS Displacement 0.000027 0.001200 YES
https://wiki.ch.ic.ac.uk/wiki/images/0/01/MD_BH3_OPT_631G_DP.LOG
Optimsed BH3 |
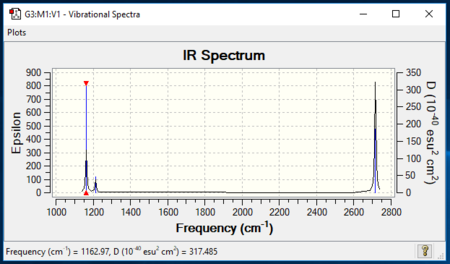
| wavenumber (cm-1) | Intensity (arbitary units) | Symmetry | IR Active? | Type |
|---|---|---|---|---|
| 1163 | 93 | A2 | yes | out-of-plane-bend |
| 1213 | 14 | E' | Slightly | In-plane-bend |
| 1213 | 14 | E' | Slight | In-plane-bend |
| 2582 | 0 | A'1 | No | Totally Symmetric Stretch |
| 2716 | 126 | E' | yes | Asymmetric Stretch |
| 2716 | 126 | E' | yes | Asymetric Stretch |
Insert IR Spectrum
Ng611 (talk) 13:28, 17 May 2019 (BST) You need to explain why you only observe 3 modes in the IR spectrum, vs the 6 in the frequency table.
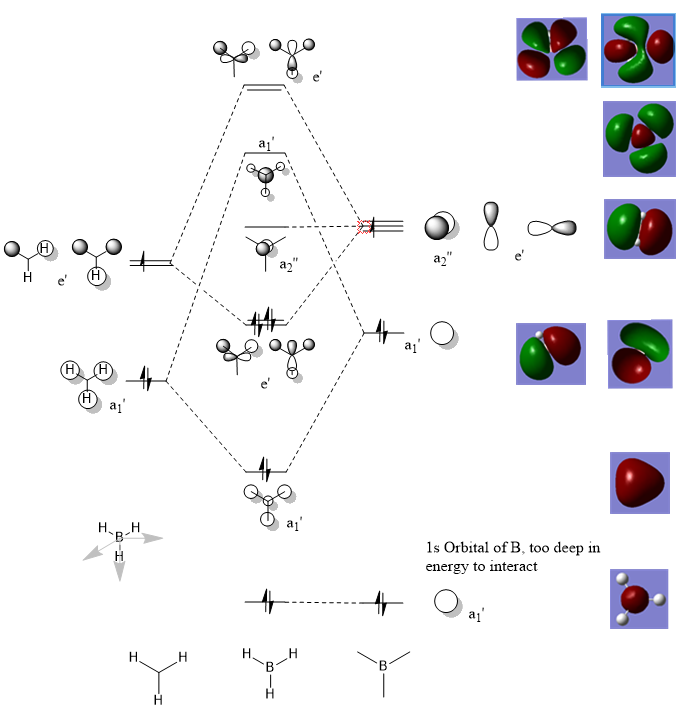
Molecular Orbital Diagram For BH3
Molecular Shape:Trigonal Planar
Point Group:D3h
MO Diagram:
Are there any significant differences between the real and LCAO MOs?
The pictorial representation of the orbitals shows the individual contributions from each of the orbitals. However, the actual orbitals are more diffuse and shows a rough shape of what we have predicted. Not as clear as to where the electron density is coming from.
Ng611 (talk) 13:30, 17 May 2019 (BST) Good MO diagram, well done. You need to be more specific in describing the differences to get the full mark for your explanation.
What does this say about the accuracy and usefulness of qualitative MO theory?
MO theory is a good way to make a prediction for the Schrodinger equation solutions of the molecular orbitals. The energy levels of the MOs are predicted in the same order as the MO diagram and the pictorial representations represent the MOs accurately.
Association energies: Ammonia-Borane
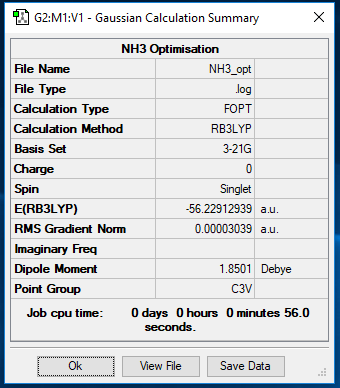
NH3
B3LYP/3-21G level
Item Value Threshold Converged? Maximum Force 0.000057 0.000450 YES RMS Force 0.000038 0.000300 YES Maximum Displacement 0.000145 0.001800 YES RMS Displacement 0.000096 0.001200 YES Predicted change in Energy=-1.132404D-08 Optimization completed. -- Stationary point found.
https://wiki.ch.ic.ac.uk/wiki/images/e/e5/NH3_OPT.LOG
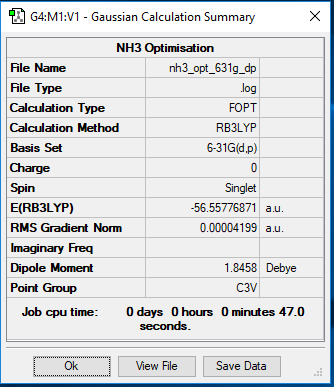
6-31G(d,p) NH3
Item Value Threshold Converged? Maximum Force 0.000060 0.000450 YES RMS Force 0.000040 0.000300 YES Maximum Displacement 0.000369 0.001800 YES RMS Displacement 0.000162 0.001200 YES Predicted change in Energy=-2.259209D-08 Optimization completed. -- Stationary point found.
https://wiki.ch.ic.ac.uk/wiki/images/c/ce/NH3_OPT_631G_DP.LOG
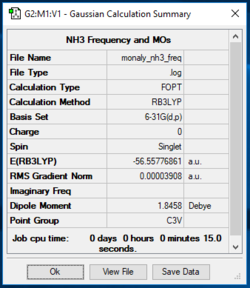
Frequency Analysis6-31G(d,p) NH3
Item Value Threshold Converged? Maximum Force 0.000056 0.000450 YES RMS Force 0.000038 0.000300 YES Maximum Displacement 0.000151 0.001800 YES RMS Displacement 0.000074 0.001200 YES Predicted change in Energy=-1.302934D-08 Optimization completed. -- Stationary point found.
Low frequencies --- -0.0125 -0.0063 -0.0022 309.9591 309.9613 369.9004 Low frequencies --- 864.2110 1685.0379 1685.0382
https://wiki.ch.ic.ac.uk/wiki/images/c/c8/MONALY_NH3_FREQ2.LOG
Optimsed NH3 |
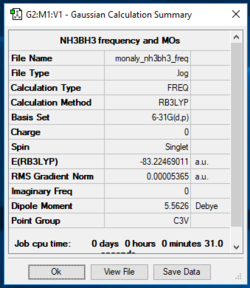
NH3BH3
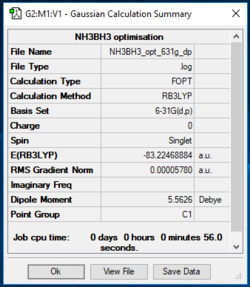
B3LYP/3-21G level, NH3BH3
Item Value Threshold Converged? Maximum Force 0.000087 0.000450 YES RMS Force 0.000032 0.000300 YES Maximum Displacement 0.000361 0.001800 YES RMS Displacement 0.000193 0.001200 YES Predicted change in Energy=-5.541366D-08 Optimization completed. -- Stationary point found.
https://wiki.ch.ic.ac.uk/wiki/images/7/76/NH3BH3_OPTIMISATION.LOG
B3LYP/B-31G level, NH3BH3
Item Value Threshold Converged? Maximum Force 0.000137 0.000450 YES RMS Force 0.000038 0.000300 YES Maximum Displacement 0.001014 0.001800 YES RMS Displacement 0.000224 0.001200 YES Predicted change in Energy=-1.121241D-07 Optimization completed. -- Stationary point found.
https://wiki.ch.ic.ac.uk/wiki/images/a/a9/NH3BH3_OPT_631G_DP.LOG
Frequency Analysis6-31G(d,p) NH3BH3
Item Value Threshold Converged? Maximum Force 0.000243 0.000450 YES RMS Force 0.000054 0.000300 YES Maximum Displacement 0.001387 0.001800 YES RMS Displacement 0.000374 0.001200 YES Predicted change in Energy=-1.934793D-07 Optimization completed. -- Stationary point found.
Low frequencies --- -0.2081 -0.0609 -0.0066 10.0986 16.5503 16.5595 Low frequencies --- 263.0248 631.3813 638.8710
https://wiki.ch.ic.ac.uk/wiki/images/a/a9/MONALY_NH3BH3_FREQ.LOG
Optimsed NH3BH3 |
Total Energies (au)
| Molecule | Total Energy (au) |
|---|---|
| NH3 | -26.61532 |
| BH3 | -56.55777 |
| NH3BH3 | -83.22469 |
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]
ΔE=E((-83.22469)-(-56.55777)+(-26.61532))
ΔE=E(-0.0516)
ΔE=-135 kJ/mol
Is the B-N bond weak, medium or strong?
Ng611 (talk) 13:32, 17 May 2019 (BST) That's for you to answer... :)
NI3
Item Value Threshold Converged? Maximum Force 0.000067 0.000450 YES RMS Force 0.000044 0.000300 YES Maximum Displacement 0.000482 0.001800 YES RMS Displacement 0.000327 0.001200 YES Predicted change in Energy=-5.632576D-08 Optimization completed. -- Stationary point found.
https://wiki.ch.ic.ac.uk/wiki/images/5/5f/MD5317_NI3_FREQ.LOG
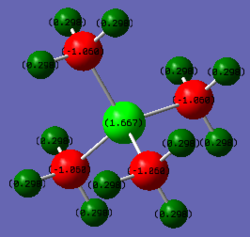
Optimsed NI3 |
Ng611 (talk) 13:33, 17 May 2019 (BST) What was the NI bond length?
Project Section
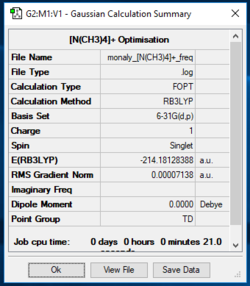
[N(CH3)4]+
[N(CH3)4]+ Optimisation 631g(d,p)
Item Value Threshold Converged? Maximum Force 0.000029 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000772 0.001800 YES RMS Displacement 0.000300 0.001200 YES Predicted change in Energy=-1.443532D-08 Optimization completed. -- Stationary point found.
https://wiki.ch.ic.ac.uk/wiki/images/4/4f/-N%28CH3%294-%2B_OPT_631G_DP.LOG
[N(CH3)4]+ Frequency Analysis
Low frequencies --- 0.0007 0.0007 0.0008 32.3845 32.3845 32.3845 Low frequencies --- 212.7850 313.4724 313.4724
Item Value Threshold Converged? Maximum Force 0.000082 0.000450 YES RMS Force 0.000066 0.000300 YES Maximum Displacement 0.000582 0.001800 YES RMS Displacement 0.000487 0.001200 YES Predicted change in Energy=-4.660485D-07 Optimization completed. -- Stationary point found.
https://wiki.ch.ic.ac.uk/wiki/images/e/e8/-N%28CH3%294-%2B_OPT_631G_DP_FREQ.LOG
Optimsed [N(CH3)4]+ |
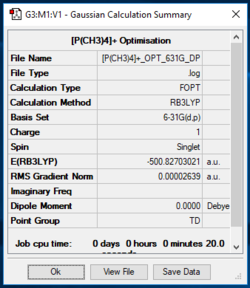
[P(CH3)4]+
[P(CH3)4]+ Optimisation 631G (d,p)
Item Value Threshold Converged? Maximum Force 0.000064 0.000450 YES RMS Force 0.000027 0.000300 YES Maximum Displacement 0.000783 0.001800 YES RMS Displacement 0.000307 0.001200 YES Predicted change in Energy=-1.676732D-07 Optimization completed. -- Stationary point found.
https://wiki.ch.ic.ac.uk/wiki/images/3/3c/-P%28CH3%294-%2B_OPT_631G_DP.LOG
Optimsed [P(CH3)4]+ |
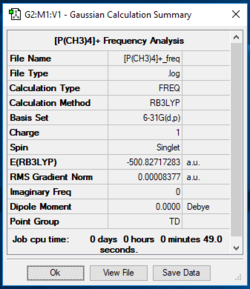
[P(CH3)4]+ Frequency Analysis
Low frequencies --- -0.0017 -0.0015 0.0009 50.3540 50.3540 50.3540 Low frequencies --- 186.2688 211.3255 211.3255
Item Value Threshold Converged? Maximum Force 0.000149 0.000450 YES RMS Force 0.000084 0.000300 YES Maximum Displacement 0.000934 0.001800 YES RMS Displacement 0.000521 0.001200 YES Predicted change in Energy=-7.780930D-07 Optimization completed. -- Stationary point found.
https://wiki.ch.ic.ac.uk/wiki/images/c/c0/-P%28CH3%294-%2B_FREQ.LOG
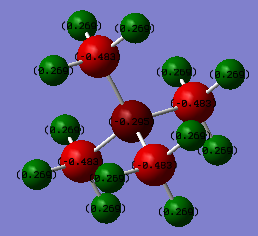
[P(CH3)4]+ and [N(CH3)4]+ Charge Distribution
| Atom | [P(CH3)4]+ | [N(CH3)4]+ | Comparison |
|---|---|---|---|
| Heteroatom (P/N) | 1.67 | -0.30 | The electronegativities of N and P are 3.04 and 2.19 respectively, P is on the third row of the PTE and N is on the second row. P having a lower electronegativity, will mean that it can stabilise a positive charge more effectively than a N. Additionally, P is in the third row which means that the atom is more diffuse, the charge density will decrease which further contributes to the stability of the positive charge. |
| C | -1.06 | -0.48 | The electronegativity of C is 2.55. This means that C is more electronegative than P, therefore there will be more electron density on the C in a C-P bond relative to the C in a C-N bond, N is more electronegative than C. However, C will still have a negative charge in the C-N bond, this is because the N is not very able to hold onto a positive charge therefore, the unit charge has to be spread across all of the atoms. |
| H | 0.30 | 0.27 | C-H there is not a big difference in these values because electronic effects seen due to electronegativity will drop rapidly with distance away from the atom. This means that the C-H bonds will be very similar. |
Ng611 (talk) 13:38, 17 May 2019 (BST) What about for atoms related by symmetry?
The formal positive charge on the N atom is a result of the Lewis Structure. The first 3 bond to the methyl groups are a covalent bond, however, due to N only having 5 valence electrons the fourth bond to the methyl group is a dative bond. This means that there is a positive charge to show the difference in the number of electrons that are expected to be seen on a neutral atom as opposed to the number of electrons on the atom in the Lewis structure. Electron deficiency. However, the positive charge is actually spread across the C atoms as well as the N atom, this increases the stability of the cation as the C is less electronegative so can stabilise a positive charge better than a N atom. Additionally, this diagram is just trying to show charges as if they can be localised to an atom however, this is not the case - an MO diagram shows the distribution of a charge in a molecule across different atoms.
Ng611 (talk) 13:38, 17 May 2019 (BST) Good answer!
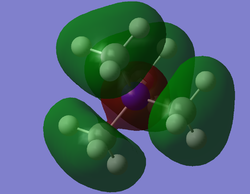
[N(CH3)4]+ Valence MOs
MO 20
MO 9
MO 10
Ng611 (talk) 13:41, 17 May 2019 (BST) Your first LCAO is good, well done. However, I don't agree with your FOs for MOs 9 and 10. You've given them s-type symmetry when there's an inversion plane (centred on the carbon), making their symmetry p type.
MO 20 is overall a bonding orbital. There are regions where there are anti-bonding interactions occurring through spaces however, overall it is bonding because the nodes are occurring on the p-orbitals of the C and N atoms. MO 10 is a bonding orbital. There are nodes only at the p-orbitals of the C atoms, the rest of the orbitals are s and belong to the N and H atoms. The p-orbitals are orientated to be in phase with both of the different s-orbitals and as there are no nodes along the bonds this is bonding. Finally, MO 9 is bonding, one node that occurs at the N due to the presence of a p-orbital. Does not have as many antibonding interactions through space either, which makes this the most bonding orbital of the three chosen.
Ng611 (talk) 13:41, 17 May 2019 (BST) Good descriptions, but it would be better to annotate these on your LCAO diagrams.