Lp1916
NH3 Molecule
Basic information
•Molecule Name: Ammonia
•Calculation Method: RB3LYP
•Basis Set: 6-31G(d,p)
•Final Energy E(RB3LYP)=-56.55776873 a.u.
•Point Group: C3V
•N-H bond distance=1.01798 Å
•H-N-H bond angle=105.741°
Item table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Optimisation LOG document
Ammonia |
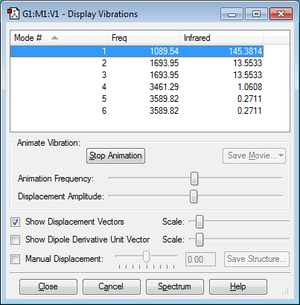
Vibrational modes analysis
•Number of vibrational modes=3N-6 where N=4. Therefore I expect the number to be 6.
•Modes number 2 and 3 and modes number 5 and 6 from picture above are degenerate.
•Modes number 1&2&3 are bond bending and modes number 4&5&6 are bond stretching.
•Mode number 4 is highly symmetrical.
•Mode number 1 is the umbrella mode.
•2 bands are expected to see in an experimental spectrum of gaseous ammonia.
Charge Analysis
•Charge on N-atom is -1.125 e
•Charge on H-atom is 0.375 e
•I expect N-atom to have negative charge due to its higher electronegativity and vice versa for H-atom
N2 Molecule
Basic information
•Molecule Name: Nitrogen
•Calculation Method: RB3LYP
•Basis Set: 6-31G(d,p)
•Final Energy E(RB3LYP)=-109.52412868 a.u.
•Point Group: D*H
•N-N bond distance=1.10550 Å
Item table
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
•Display Vibrations-Frequency = 2457.33
H2 Molecule
Basic information
•Molecule Name: Hydrogen
•Calculation Method: RB3LYP
•Basis Set: 6-31G(d,p)
•Final Energy E(RB3LYP)=-1.17853936 a.u.
•Point Group: D*H
•H-H bond distance=0.74279 Å
Item table
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
•Display Vibrations-Frequency = 4465.68
E(NH3)= -56.55776873 a.u.
2*E(NH3)= -113.1155375 a.u.
E(N2>/sub>)= -109.52412868 a.u.
E(H2)= -1.17853936 a.u.
3*E(H2)= -3.53561808 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579704 a.u. = -146.50 kJ/mol
NH3 is more stable as it has lower energy.
Project molecule
H2CO Molecule
Basic information
•Molecule Name: Formaldehyde
•Calculation Method: RB3LYP
•Basis Set: 6-31G(d,p)
•Final Energy E(RB3LYP)=-114.50319933 a.u.
•Point Group: CS
•C=O bond distance=1.20676 Å
•C-H bond distance=1.11056 Å
•H-C-H bond angle=115.219°
•H-C=O bond angle=122.386°
Item table
Item Value Threshold Converged? Maximum Force 0.000197 0.000450 YES RMS Force 0.000085 0.000300 YES Maximum Displacement 0.000270 0.001800 YES RMS Displacement 0.000149 0.001200 YES
Optimisation LOG document
Methanal |

Vibrational modes analysis
•Number of vibrational modes=3N-6 where N=4. Therefore I expect the number to be 6.
•All the modes above are NOT degenerate.
•Modes number 1&2&3 are bond bending of C-H bonds, mode number 4 has both bending (C-H) and stretching (C=O) character and modes number 5&6 are bond stretching (C=O).
•Modes number 3&5 are quite symmetrical.
•6 bands are expected to see in an experimental spectrum of methanal.
Charge Analysis
•Charge on O-atom is -0.494 e
•Charge on C-atom is 0.221 e
•Charge on H-atom is 0.137 e
•I expect O-atom to have negative charge since it has the highest electronegativity value among the three atoms.
Some Molecular Orbitals

This is 1σ non-bonding orbital from Oxygen 1s orbital. It has deeper energy as Oxygen is more electronegative.
This is 2σ non-bonding orbital from Carbon 1s orbital. Its energy is quite higher comparing with that of oxygen.Χ(O) = 3.5 where Χ(C) = 2.5 which is much lower than that of Oxygen. Therefore Energy is higher.

This is the bonding orbital of carbon 2p and oxygen 2p orbitals forming C-O sigma bond. The reason that the energy of two p orbital combining is lower is probably due to orbital mixing.1 [1]

This is the bonding orbital of C-H sigma bond comprising of carbon 2s orbital and hydrogen 1s orbital.1 [1]

This is the asymmetric C-H sigma bond.1 [1]
Comparison with Literature
The molecular orbital of methanal is very complicated and I searched for literature results to validate my conjecture. The general appearance of the molecular orbitals looks perfectly fine comparing to the literature results. However, I found the explanation quite confusing. I will ask Mr. Vilar for further details and above partially comes from the literature. Image below shows the MO in increasing order of energy. 1 [1]



