Leroy Project
Computational Laboratory Mini Project
Cis-Platin and its Derivatives
First discovered in 1845 by Michel Peyrone[1], cis-diamminedichloroplatinum(II)[2] is a chemotherapy drug widely used for treatment of cancers such as sarcomas and lymphomas. It wasn't until the early 1960's that its medicinal properties were revealed, when it was found to show effective inhibition of Escherichia Coli. Further research lead to widescale production of the compound for use in global cancer treatment. Other, more common, names for the drug include 'cis-platin', cis-platinum and CDDP.
In this project, the synthesis of the drug will be analysed followed by considerations into the isomer of the drug - trans-platin.
Synthesis
The following is the reaction scheme for the synthesis of cisplatin[3]:
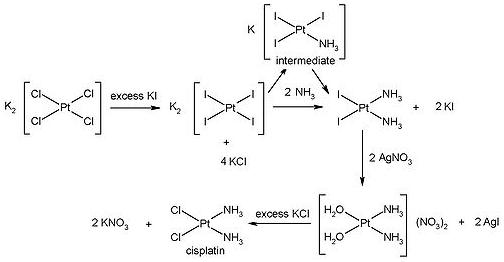
The starting reagent is [PtII(Cl4)]2-, this is reacted with excess KI to form [PtII(I4)]2-. This stage crutial in the synthesis of the cis-isomer, since I- has a greater trans effect than Cl-. Thus upon reaction of [PtII(I4)]2- with NH3, the additon of the second NH3 group is such that it is trans to I- and therefore cis to the initial NH3 ligand.
I- ligands exhibit greater stability than Cl- ligands and thus cannot be directly substituted. To overcome this, the I- ligands are replaced with H2O which is a very labile ligand and is consequently easily displaced by Cl-. The resulting product is cisplatin.
Square planar PtII complexes show very slow ligand-exchange reactions, this explains why cis-isomers do not readily interconvert to the trans-isomer in solution.
Clinical Effect
Cisplatin is administered intravenously with saline solution, once in the bloodstream the drug gradually diffuses into the cells. Bioavailibilty of the drug/uptake is rather poor, since 50-70% is excreted from the body within 24 hours.
Design of the drug is such that little chemistry occurs the with the blood proteins and is specific to tissue proteins. This is achieved by the presence of the labile Cl- ligands.
In the bloodstream there is a high concentration of Cl- ions and thus the Cl ligands on the drug remain relatively inert. However once absorbed into the target cell, the Cl- concentration significantly decreases, rendering the Cl- ligand open to attack and fundamentally interconversion to its activated hydroxylated form:
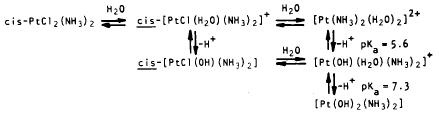
When the complex becomes hydroxylated it has the ability to readily bind to the lewis acidic nitrogen groups of Adenine, Guanine and Cytosine nucleobases that are present on DNA. This is because the hydroxyl ligand is extremely labile and easily displaced.
Once bound to the DNA the second hydroxyl group enables the formation of a 1,2-intrastrand adduct which ultimately results in apoptosis of the target cell.
This where the cis and trans isomers show different clinical effect, and is the reason why cisplatin is the most effective isomer. Since transplatin undergoes the same chemistry as cisplatin, excluding the formation of the 1,2-intrastrand adduct, instead a less favourable 1,3-intrastrand adduct is formed. This 1,3-intrastrand adduct is highly susceptible to the Nucleotide Excision Repair mechainsm, thus DNA apoptosis is prevented.
Calculations and Determination of Basis sets
Given the background relating to the synthesis and clinical effects of cisplatin and its derivatives, it was decided to:
- Perform optimisation calculations and frequency analysis for the structures [PtII(Cl)2(NH3)2] and [PtII(I)2(NH3)2], to help explain the choice of Cl- as the ligand present on cisplatin.
- Optimise the transplatin isomer and make comparisons of frequency and MO analysis with that of cisplatin.
By examination of all the complexes that are to be investigated, it is apparent that each of the complexes contains a heavy atom. Previous experience of optimisation calculations involving heavy atoms lead to the use of LANL2MB and LANL2-DZ basis sets - with the latter showing a higher degree of complexity, thus providing more accurate results.
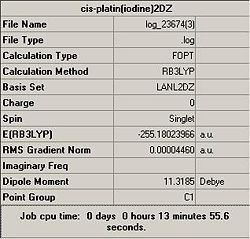
Preliminary caculations, using both basis sets, for the optimisation of cis-diamminediiodoplatinum(II) where undertaken in order to determine which basis set could be used effectively throughout the project.
B3LYP calculation method was used throughout the project.


 |
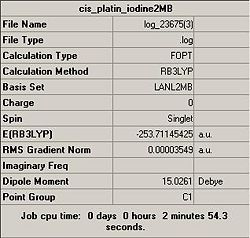 |
Clearly both the optimisation calculations were successful, this was supported by a convergence in the output file. As expected, the LANL2-DZ basis set provided a more energetically stable complex, and the calculation time for this more accurate basis set proved to be feasible. Thus it was concluded that the LANL2-DZ basis set and B3LYP calculation methods were to be used for all future calculations.
Comparisons of cis-[PtII(Cl)2(NH3)2] and cis-[PtII(I)2(NH3)2]
As previously discussed, the synthesis of cisplatin involves the production of cis-[PtII(I)2(NH3)2] prior to the formation of cis-[PtII(Cl)2(NH3)2]. Frequency analysis of both molecules will be used to illustrate why the cis-[PtII(I)2(NH3)2] has to be converted to cis-[PtII(H2O)2(NH3)2] prior to conversion to cis-[PtII(Cl)2(NH3)2].
Optimisation calculations for both molecules were conducted using the method (B3LYP) and basis set (LANL2-DZ)previously established:

 |
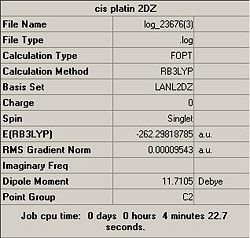 |
Analysis of the summary files indicates that cis-diamminedichloroplatinum is considerably more thermodynamically stable than cis-diamminediiodoplatinum, this provides one reason why chlorine ligands are used, since the increased stability willl reduce the risk of unwanted chemical reaction within the body.
However the dipole moment for the chloro monomer is greater than that of the iodo, indicating a weaker bond and therefore more labile ligand. Which is highly beneficial given the reaction pathway that has to be followed in order to show clinical effect upon the target cell - i.e. hydroxylation.
To further investigate the strength of the Pt-I bond in relation to the Pt-Cl bond, a frequency analysis was performed with the same method (B3LYP) and basis set (LANL2-DZ) as that used for the optimisation.
The results were as follows:
| Description | Frequency (cm-1) | Intensity |
|---|---|---|
| In plane symmetric bending/scissoring of iodine ligands. | 74 | 0.06 |
| In plane asymmetric stretching of the iodine ligands. | 168 | 9.39 |
| In plane symmetric stretchin gof the iodine ligands. | 176 | 2.39 |
| Description | Frequency (cm-1) | Intensity |
|---|---|---|
| In plane symmetric bending/scissoring of chlorine ligands. | 135 | 0.06 |
| In plane asymmetric stretching of the chlorine ligands. | 320 | 9.39 |
| In plane symmetric stretchin gof the chlorine ligands. | 330 | 2.39 |
Vibrational frequencies are all higher for the Pt-Cl than the Pt-I bond, indicating a stronger bond. This is in contrast to the data provided by the dipole moments of the complexes, but supports the energy values obtained for the complexes.
Thus the main reason for chlorine being a more labile ligand than iodine is its polarity and ability to behave as a anion in solution.
References and Citations
- ↑ Cis-Platin {(http://www.cisplatin.org )}
- ↑ Cisplatin: synthesis, antitumour activity and mechanism of action{(http://www.springerlink.com/content/y70578940g756031/fulltext.pdf )}
- ↑ cisplatin {(http://en.wikipedia.org/wiki/Cisplatin )}
