Kt916
Y2 Inorganic Computational Lab
EX3 Section
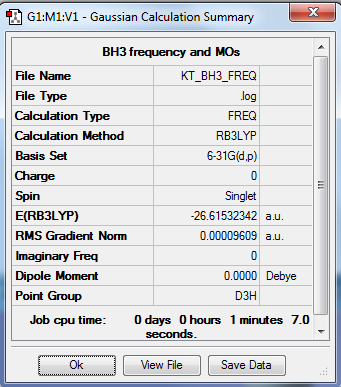
BH3
Method: RB3LYP Basis Set: 6-31G(d.p)
Item Value Threshold Converged?
Maximum Force 0.000192 0.000450 YES
RMS Force 0.000126 0.000300 YES
Maximum Displacement 0.000763 0.001800 YES
RMS Displacement 0.000500 0.001200 YES
Frequency analysis log file File:KT BH3 FREQ.LOG
Low frequencies --- -0.1082 -0.0045 -0.0006 46.3499 46.3501 47.3456 Low frequencies --- 1163.7050 1213.6299 1213.6302
BH3 Molecule |
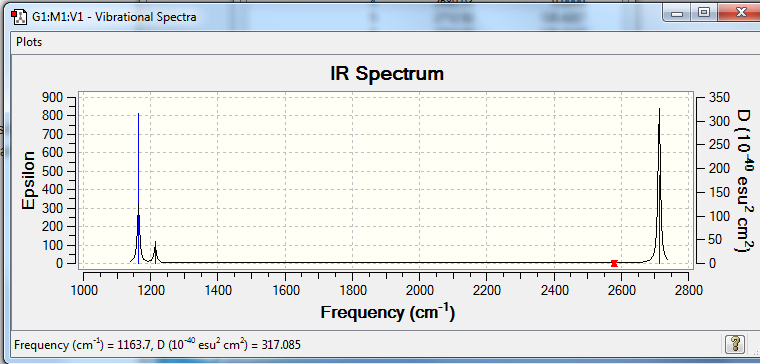
Vibrational spectrum for BH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1164 | 92 | A2ˈˈ | yes | out-of-plane bend |
| 1214 | 14 | E' | very slight | bend |
| 1214 | 14 | E' | very slight | bend |
| 2580 | 0 | A1ˈ | no | symmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
| 2713 | 126 | E' | yes | asymmetric stretch |
For an stretch to be IR active, it must, after vibration have a change in dipole moment in which the stretch at 2580cm-1, the symmetric stretch does not. The two vibrations corresponding to 1214 and 2713-1, have 2 vibrations that are degenerate, hence they are superimposed on the IR spectra. Hence only 3 peaks are shown on the final spectra, they are 1163, 1214 and 2712 cm-1
MO Diagram
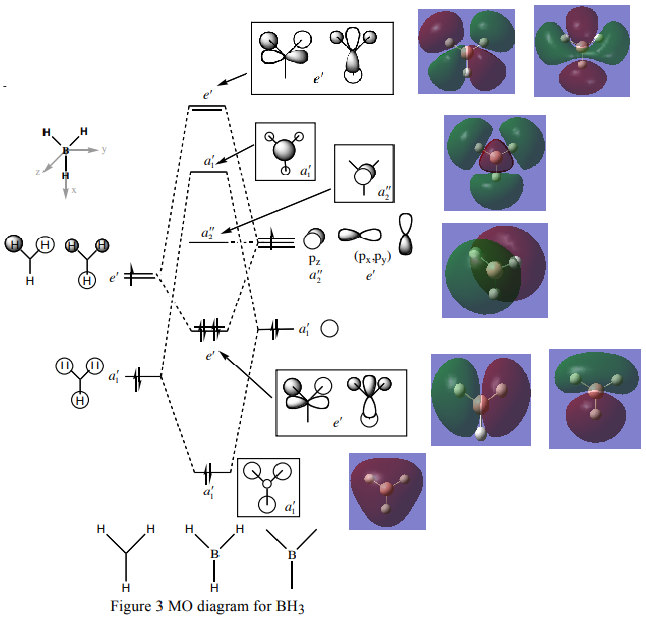 Taken from http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf, accessed on 08/05/2018
Taken from http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf, accessed on 08/05/2018
The Linear Combination of Atomic orbitals have correctly predicted the phase and the nodes of the real MO's, however the LCAO orbitals do not encompass the whole molecule and are a bit misaligned in regards to the electron densities. The shapes of the MO's are pretty close, they are, however calculated using the IPA (independent Particle approximation) hence to not take into account the V term (The interactions between the electrons) so a slight difference is expected.
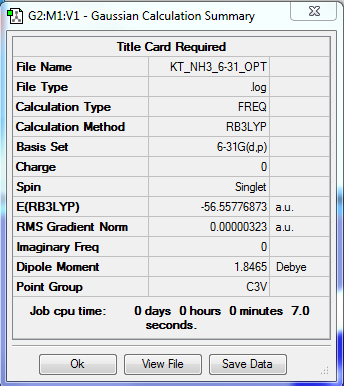
NH3
Method: RB3LYP Basis Set: 6-31G(d.p)
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000014 0.001800 YES
RMS Displacement 0.000009 0.001200 YES
Frequency analysis log file File:KT NH3 6-31 OPT.LOG
Low frequencies --- -0.0130 -0.0018 0.0013 7.1034 8.1048 8.1051 Low frequencies --- 1089.3834 1693.9368 1693.9368
NH3Molecule |
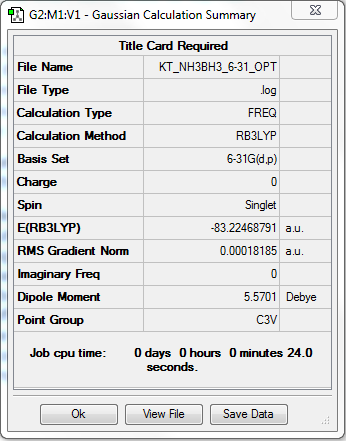
NH3BH3
Method: RB3LYP Basis Set: 6-31G(d.p)
Item Value Threshold Converged?
Maximum Force 0.000179 0.000450 YES
RMS Force 0.000097 0.000300 YES
Maximum Displacement 0.001586 0.001800 YES
RMS Displacement 0.000696 0.001200 YES
Frequency analysis log file File:KT NH3BH3 6-31 OPT.LOG
Low frequencies --- -0.0280 -0.0097 -0.0049 10.4098 10.4563 24.2385 Low frequencies --- 265.0508 634.1235 639.9020
NH3Molecule |
Energy Calculations
E(NH3) = -56.55777 Eh = -141394 kJ/mol
E(BH3) = -26.61532 Eh = -66538 kJ/mol
E(NH3BH3) = -83.22469 Eh = -208062 kJ/mol
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] = -0.05160 Eh = -129 kJ/mol
I would say that the bond is around medium strength as expected for a bond with an ionic component. (Electronegativity difference between B and N = 1.0 ). Weaker than a C-C bond (350 KJ/mol) yet stronger than typical Hydrogen bonds (20 KJ/mol).
Smf115 (talk) 17:13, 23 May 2018 (BST)Correct calculation methods, however, the answer has been rounded incorrectly to kJ/mol and literature values should be referenced.
BBr3 pseudo-potentials analysis
Method: RB3LYP Basis Set: 6-31G(d.p), (B), LanLDZ (Br) (manually specified pseudo-potentials)
Item Value Threshold Converged?
Maximum Force 0.000008 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000037 0.001800 YES
RMS Displacement 0.000018 0.001200 YES
Predicted change in Energy=-4.301212D-10
Optimization completed.
-- Stationary point found.
Frequency analysis log file File:KT BBR3 5 FREQ.log
Dspace link DOI:10042/202377
Low frequencies --- -2.3055 -0.0029 -0.0018 0.0774 0.7534 0.7534 Low frequencies --- 155.9402 155.9405 267.6894
BBR3Molecule |
Project Section: Aromaticity
Borazine
Item Value Threshold Converged? Maximum Force 0.000194 0.000450 YES RMS Force 0.000061 0.000300 YES Maximum Displacement 0.000293 0.001800 YES RMS Displacement 0.000093 0.001200 YES
Low frequencies --- -0.0303 -0.0150 -0.0036 3.2025 3.2053 3.7578 Low frequencies --- 289.7201 289.7209 404.4099
Borazine |
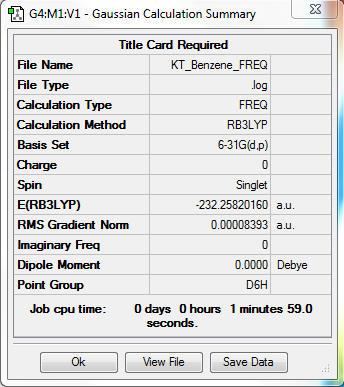
Benzene
Item Value Threshold Converged? Maximum Force 0.000194 0.000450 YES RMS Force 0.000084 0.000300 YES Maximum Displacement 0.000767 0.001800 YES RMS Displacement 0.000328 0.001200 YES
Low frequencies --- -16.9682 -14.6636 -14.6636 -0.0055 -0.0055 -0.0009 Low frequencies --- 414.1239 414.1239 620.9400
Benzene |
Charge Distributions
| Benzene | Borazine |
|---|---|
 |
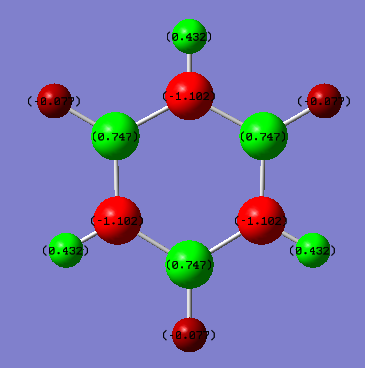
|
For Benzene, the charge distribution for all the Carbon's and Hydrogen's are the same, which is to be expected under the D6H symmetry as all of the C and N are identical to one another. Carbon's Electronegativity is 2.55 while Hydrogen's is 2.20, leading the the electron density in the C-H bond to be drawn to the Carbon, hence -2.39 Db charge and +2.39 Db charge for Carbon and Hydrogen respectively. In addition, there is no dipole moment in the molecule (It all cancels out due to the center of inversion present in the center of the flat ring).
With the introduction of Boron and Nitrogen instead of Carbon in Borazine, there is a reduction in symmetry from C6 to D3H. Looking at the B-N bonds, the more electronegative Nitrogen (3.04) compared to Boron (2.04) means the charges at N are -1.1042 Db and at B are +0.747 Db. In addition even H (electronegativity 2.20) is greater than Boron, leaving hydrogen with a charge of -0.077 Db while the N-H bond Hydrogens are at +0.432 Db. In this case, according to the Jmol images, the Borazine is planar with a center of inversion so there is no net dipole.
Smf115 (talk) 17:16, 23 May 2018 (BST)Great charge analysis with both the symmetry and electronegativity used to explain the distributions. Make sure that the same colour range is used to highlight the differences in the charge distribution across the molecule.
Comparing MOs
Smf115 (talk) 17:12, 23 May 2018 (BST)Great MO comparison with mention to symmetry elements and correct description of the orbital type and character.
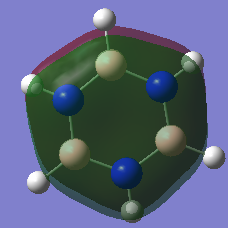
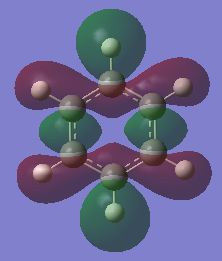
Aromaticity Discussion
| Benzene | Borazine | |
|---|---|---|
 |
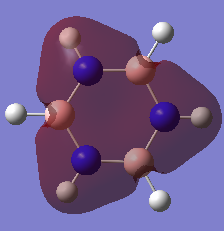 |
MO 7 |
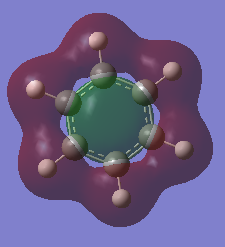 |
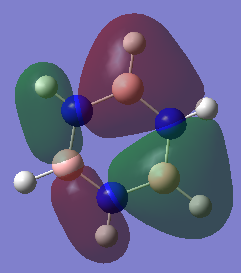 |
MO 12 |
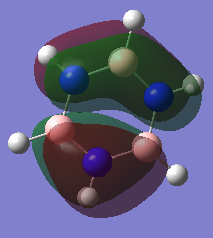
The basic concepts of aromaticity stems from the delocalisation of π electrons over multiple carbon atoms and is usually represented by the drawing of 6pz p orbitals on a benzene ring. After the computation of the molecules Benzene and Borazine, 3 bonding and 3 anti-bonding π MOs along with many σ MOs were found from the Liunear combination of atomic orbitals. MO 17 (as shown above) pretty much represents the basic picture of aromaticity of p orbital overlap, with 2 lobes above and below the ring giving rise to aromatic traits (Equal bond lengths, Resonance stabilization energies, Pi ring currents). However, it must be noted that there are 2 other π bonding MOs (20 and 21) which also shows the spreading out of electron density across the carbon molecules (albeit with some nodes) though a bit more complex and not as straight forward.
The concept of overlapping pz AOs does not give the whole story of aromaticity. This is due to 2 reasons. One being the fact that the other 2 bonding π bonding MOs were not included in the basic explanation of aromaticity. The other, is due to ignoring the σ orbital contributions to aromticity at all. Literature states that the role of σ-electron structure may be of importance as well and it is the vital to explain the more detailed picture of aromaticity. Computational analysis of MOs via LCAO shpws 2 σ orbitals for Benzene (7 and 12) which contribute to the spreading out of electron density across the carbon bonds. In Borazine, however only has 1 (MO 7), therefore the σ framework actually can be used to explain why Borazine is less aromatic than benzene (Lower resonance stabilization energy) and also why Borazine is generally more reactive.
Smf115 (talk) 17:11, 23 May 2018 (BST)Excellent discussion of the concept of aromaticity in terms of the MOs just visualised and of the contribution from other pi- and sigma-orbitals to the edelocalisation of electron density. To improve, the key concepts of aromaticity such as Huckel's rule could have been mentioned more and the literature stated should be referenced.
Smf115 (talk) 17:11, 23 May 2018 (BST)A very good project section and overall report.