Kevlar
Kevlar
| Physical properties | |
|---|---|
| Density | 1.44g/cm3 |
| Water Absorption | 3.50% |
| Moisture Absorption | 3.50% |
| Mechanical properties | |
| Tensile Strength, Ultimate | 3GPa |
| Tenacity | 2.08N/tex |
| Elongation at Break | 2.40% |
| Tensile Modulus | 112GPa |
| Poissons Ratio | 0.360 |
| Thermal Properties | |
| Specific Heat Capacity | 0.340J/g-K (at 25°C) |
| Thermal Conductivity | 0.0400W/m-K |
| Maximum Service Temperature, Air | 149°-177°C |
| Shrinkage | 0.100% (at 100°C in water and at 177°C in air) |
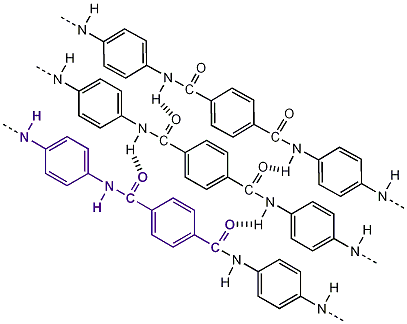
Kevlar is a para-aramid (of which aramid is an abbreviation for aromatic polyamide). Its chemical composition is poly para-phenyleneterephtalamide. The aramid ring in Kevlar gives its thermal stability, while its para structure contributes to its high tensile strength and modulus. Kevlar belongs to the group polyamides and the nylon family, but to its properties, Kevlar is better known as mentioned above, a para-aramid. Nylon for example, as an inferior strength when compared to Kevlar.
There are three different grades of Kevlar: Kevlar-29, Kevlar-49 and Kevlar-149. They have different average molecular chain length and vary in their physical properties only slightly. Kevlar-49 is the most common type of Kevlar cloth used.
Synthesis
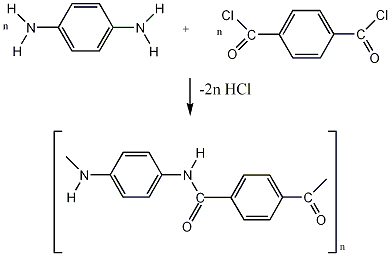
Kevlar is made from the polycondenstation reaction of 1,4-phenylenediamine and terephthaloyl chloride monomers at low temperature in amide solvents .i.e. dimethylacetamide, N-methylpyrrolidinone (NMP), hexamethyphosphoric triamide (HPT) and/or tetramethylurea. This reaction gives a hydrochloric acid as a by-product. The cost of Kevlar production is quite expensive because of the polymer’s insolubility and the use of concentrated (toxic) sulphuric acid during manufacturing.
Tensile Strength
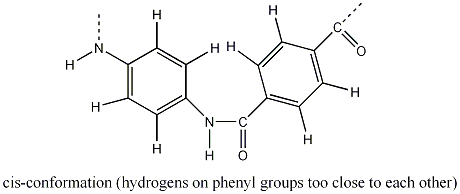
The polymer Kevlar has a tensile strength of 3.6-4.1 GPa, making it five times stronger than an equal weight of steel. This is because it has a highly linear structure and hence, is crystalline. This is due to the para-linking of the large benzene groups, which sterically hinder the Kevlar polymer chain from the more unfavourable cis-conformation of the C-N bonds. In the cis-conformation of the C-N bonds, the phenyl hydrogens are too close to each other.
The formation of hydrogen bridging bonds between the oxygen and the hydrogen atoms of adjacent and parallel polymer chains also contributes to Kevlar’s great tensile strength as well as its high structural rigidity.
Kevlar moreover, has a low electrical conductivity, high resistance to chemicals and cuts, and is also resistant to flame.
Applications
These are some of the material applications of Kevlar:
- In cables and ropes.
- In bullet-proof vests and helmets.
- In belts and hoses for industrial uses.
- As composites for body parts of aircrafts, boats and sporting equipments, like the windsurfing sails.
- In fiber-optic cables.
- In brake pads, clutch linings and gaskets.
- As adhesives, sealants and coatings.
- It is also used as a replacement for asbestos, which is carcinogenic.
References
- http://www.matweb.com/search/DataSheet.aspx?bassnum=PDUKEV29&ckck=1
- http://www.azom.com/details.asp?ArticleID=1992
- http://composite.about.com/od/aboutcompositesplastics/l/aa050597.htm
- http://www.everything2.com/index.pl?node=kevlar
- http://www2.dupont.com/Kevlar/en_US/
- http://www.pslc.ws/macrog/aramid.htm