Kemiwiki2
Inorganic computational Lab 2
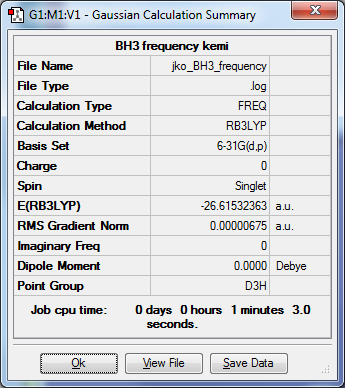
BH3
My BH3 Frequency Media:JKO_BH3_FREQUENCY.LOG
Optimized BH3 molecule |
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000014 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000053 0.001800 YES RMS Displacement 0.000027 0.001200 YES
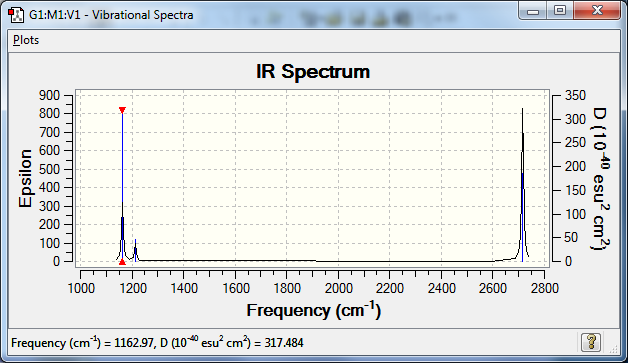
== BH3 Vibrations ==
Mode Wavenumber( /cm) IR Active? symmetry type 1 1162 yes A2" out of plane bend 2 1213 weak E' asymmetric stretch 3 1213 weak E' asymmetric stretch 4 2582 0 A1' symmetric stretch 5 2715 yes E' asymmetric stretch 6 2715 yes E' asymmetric stretch
Low frequencies --- -7.5936 -1.5614 -0.0055 0.6514 6.9319 7.1055
Low frequencies --- 1162.9677 1213.1634 1213.1661
Vibrational spectrum of BH3
There are fewer than expected vibrational peaks in the IR spectrum as not all of the vibrations are IR active so do not appear on the spectra, and some vibrations are degenerate therefore some peaks are caused by more than one vibration.
Ng611 (talk) 20:17, 15 May 2018 (BST) Good analysis! Adding the intensities of the IR modes to your table would have improved this section further.
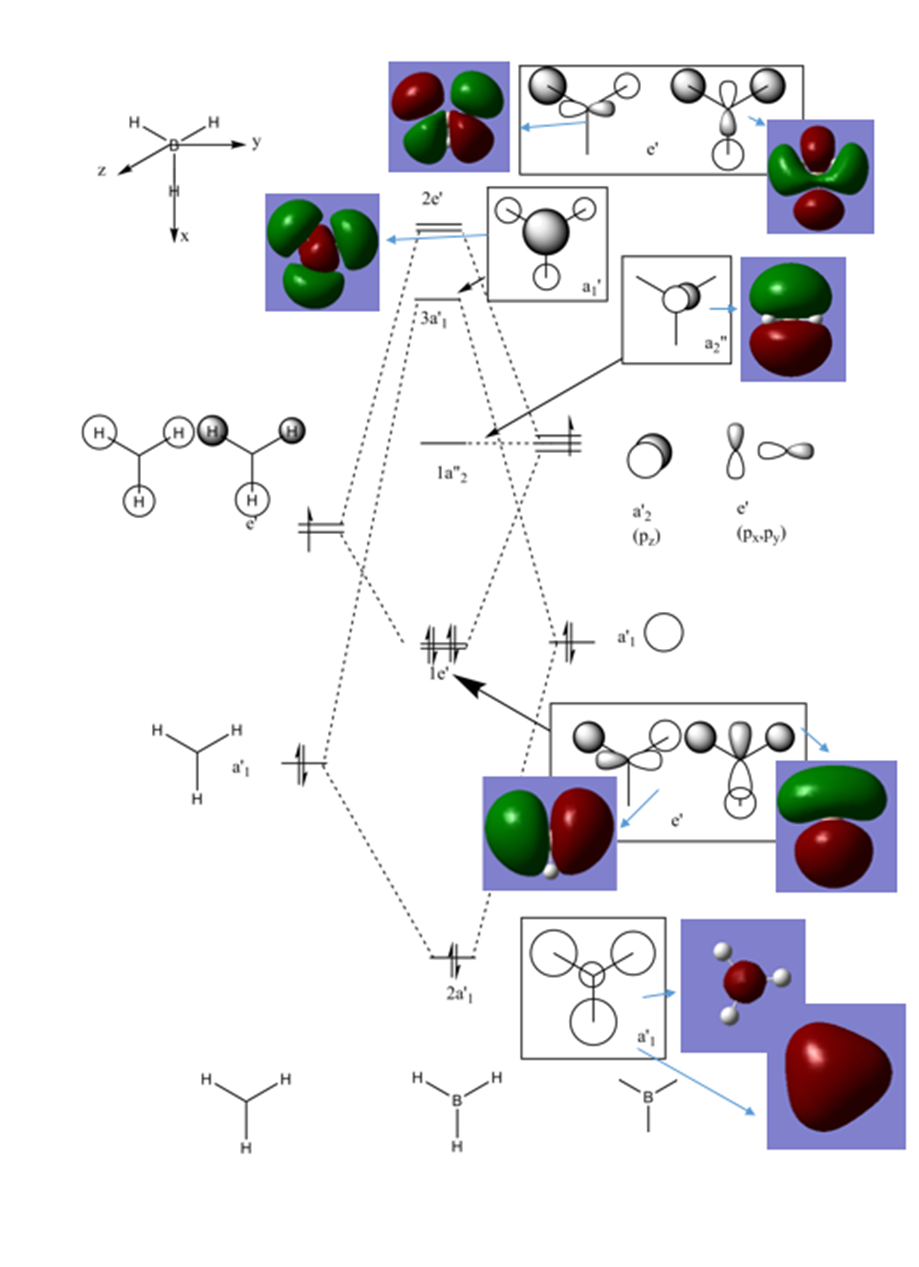
My full Molecular Orbital diagram
The LCAOs are relatively accurate as it is clear to see how they correspond to the real MOs. The only evident flaw was that
when looking at the 3a'1 orbital the LCAO would suggest that the boron atom has a greater contribution and is higher in energy,
however in the real MO diagram the hydrogen orbitals have a greater contribution, suggesting that they are higher in energy.
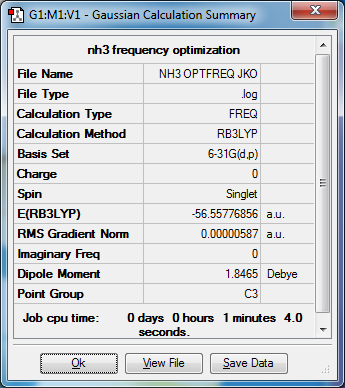
My NH3 Molecule
B3LYP/6-31G(d,p)
Optimized NH3 molecule |
Item Value Threshold Converged? Maximum Force 0.000013 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000039 0.001800 YES RMS Displacement 0.000013 0.001200 YES
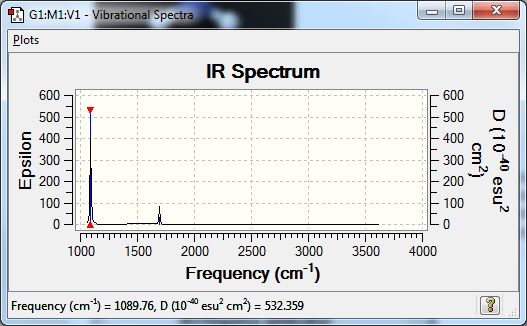
Vibrations
Low frequencies --- -8.5646 -8.5588 -0.0044 0.0454 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
Mode Wavenumber IR active? symmetry type? 1 1089 yes A1 out-of-plane bend 2 1694 slightly E bend 3 1694 slightly E bend 4 3461 no A1 symmetric bend 5 3589 no E asymmetric bend 6 3589 no E asymmetric bend
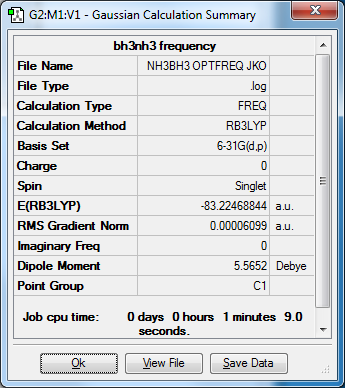
My BH3NH3 Molecule
Optimized BH3NH3 molecule |
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000114 0.000450 YES RMS Force 0.000061 0.000300 YES Maximum Displacement 0.000639 0.001800 YES RMS Displacement 0.000365 0.001200 YES
Low frequencies --- -10.6991 -0.0007 -0.0006 0.0004 19.2888 43.1695 Low frequencies --- 266.2061 632.1749 638.3865
Calculating Energies
E(NH3)= -56.55776856 (a.u) E(BH3)= -26.61532363 (a.u) E(NH3BH3)= -83.22468844 (a.u) ΔE = E(NH3BH3) - [E(BH3) + E(NH3)] ΔE = -83.22468844 -(-26.61532363 + -56.5776856) (a.u) ΔE = -0.05159625 (A.U) ΔE = -135.5 kj/mol is the association energy
This bond appears to be weak as the carbon-carbon bond has an association energy of -618.13 kj/mol ( reference: https://notendur.hi.is/agust/rannsoknir/papers/2010-91-CRC-BDEs-Tables.pdf accessed 03/05/2018) and this is not a particularly strong bond, yet has an association energy of almost triple of what was calculated for the N-B bond.
Ng611 (talk) 18:10, 17 May 2018 (BST) Interesting comparison, is this for a C-C (single) or a C=C (double) bond? Remember to cite your bond values from a textbook, databook, or paper wherever possible.
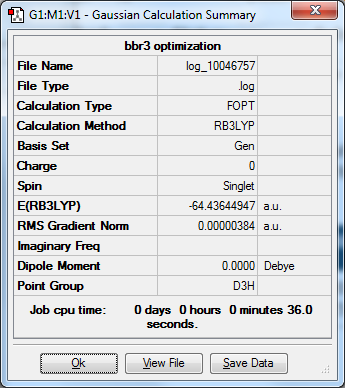
= BBr3 results
Optimized BBr3 molecule |
Item Value Threshold Converged?
Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000024 0.001200 YES Predicted change in Energy=-4.086283D-10
My initial Optimization: DOI:10042/202321
My frequency optimization : DOI:10042/202319
Part 2 and 3 - looking at Aromaticity
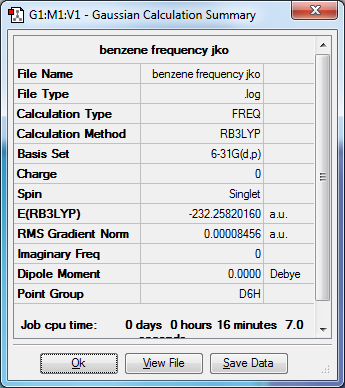
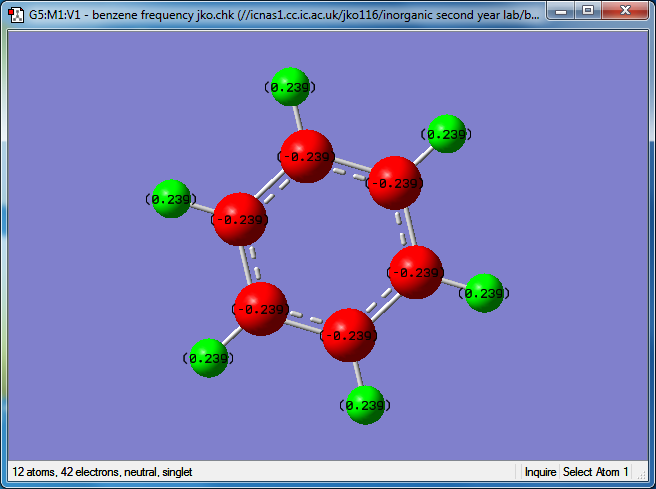
Benzene
Media:Benzene frquency jko.png.LOG
Optimized Benzene molecule |
Item Value Threshold Converged? Maximum Force 0.000187 0.000450 YES RMS Force 0.000091 0.000300 YES Maximum Displacement 0.000822 0.001800 YES RMS Displacement 0.000358 0.001200 YES
Low frequencies -13.7905 -13.0302 -11.6597 -0.0001 0.0004 0.0006 Low frequencies --- 414.0709 414.1916 620.9709
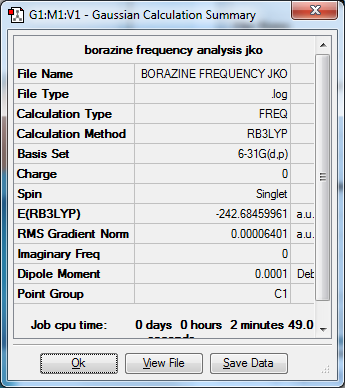
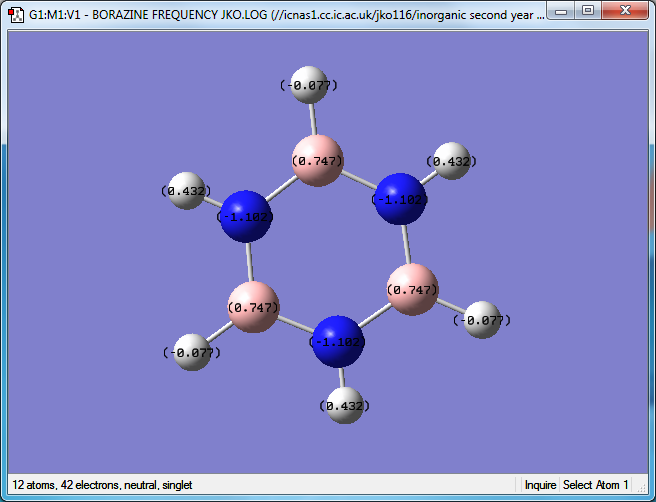
Borazine
Media: BORAZINE FREQUENCY JKO.LOG
Optimized Borazine molecule |
Item Value Threshold Converged? Maximum Force 0.000202 0.000450 YES RMS Force 0.000064 0.000300 YES Maximum Displacement 0.000301 0.001800 YES RMS Displacement 0.000106 0.001200 YES
Low frequencies --- -4.0457 -0.0006 0.0004 0.0008 7.3733 9.4017 Low frequencies --- 289.5783 289.7121 404.3407
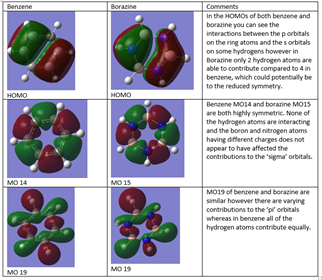
Comparing Benzene and Borazine
Borazine
Ng611 (talk) 18:11, 17 May 2018 (BST) Remember to use a colour scale!
N atom charge: -1.102
B atom charge: 0.747
BH H atom charge: -0.077
NH H atom charge: 0.432
Benzene
C atom charge: -0.239
H atom charge: 0.239
Within benzene all of the hydrogen atoms carry the same charge, as they are all connected to a carbon atom. In the borazine molecule the hydrogen atoms bonded to the nitrogen carry a positive charge while those attatched to a boron atom carry a negative charge. This is because nitrogen is more electronegative than hydrogen while boron is less electronegative than hydrogen. The charge separations are much greater in borazine compared to benzene as nitrogen is much more electronegative than carbon, and boron is less electronegative than hydrogen, meaning that the bonds are more polarised
Ng611 (talk) 20:21, 15 May 2018 (BST) Good! Remember to also explain that atoms related by symmetry will have identical charges and that the sum of all partial charges is one.
Aromaticity
Eric Hűckel deduced four guiding 'rules' to help determine whether or not a system is aromatic. Firstly, the molecule needs to be cyclic. He also stated that the molecule should be planar, and fully conjugated with parallel p orbitals on each atom in the ring system. The final rule is that the molecule must have 4n+2 pi electrons, where an is any non zero positive integer. Both Benzene and Borazine appear to follow these rules, however their molecular orbitals are differing.
Aromaticity is not in fact solely determined by these four rules. There are cases in which non planar molecules are able to show aromaticity, for example benzene bends in a lattice crystalline structure to a chair conformation due to more favourable interactions. Also, some scientists have suggested the idea that the sigma bonds have an influence on aromaticity, especially in more modern systems such as fullerenes and metallobenzenes. Huckels rules do not account for this and therefore cannot be used in isolation to determine whether or not a molecule will display aromaticity.
Both Benzene and borazine adhere to the Huckels' rules stated above, however the molecular orbital diagrams differ significantly. Benzene follows the 4n+2 electron rule using one electron from each carbon atom, and borazine also does due to the lone pairs present on the nitrogen that can donate into the empty p orbitals on boron. Both molecules are planar and cyclic, forming a conjugated system. The obvious difference between benzene and borazine is that instead of carbon, borazine contains alternating nitrogen and boron atoms. There is a big difference in charge distribution which makes it harder to delocalise the system as the bonds become more polarised in the system. Nitrogen holds onto the electrons more than boron as it is a more electronegative atom, which makes it harder to generate the delocalised system.
Ng611 (talk) 18:18, 17 May 2018 (BST) You allude to Huckel's rules creating an incomplete picture of aromaticity, which is absolutely true, well done. To that extent does delocalization dictate aromaticity - is it just delocalization of pi orbitals or are there other factors? Can we confirm the presence of aromaticity experimentally? If so, is there any literature that does this?
Ng611 (talk) 20:24, 15 May 2018 (BST) A solid report. Layout somewhat messy but most of the content was there. Some missing data however. Section 1 was excellent. The explanation of aromaticity in section 2 was a good start; some more discussion (how do experimental studies allow us to assess aromaticity) would have improved it even further.