Kc8
Module 1
Modelling using Molecular Mechanics
The Hydrogenation of Cyclopentadiene Dimer
At room temperature, cyclopentadiene dimerises to form the endo dimer rather than the exo dimer. The reasons for the stereospecificity of this reaction can be investigated by comparing the relative energies of the two products through Molecular Mechanics. Using the MM2 force field, the geometries of the two isomers their relative stabilities was computed.
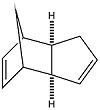 |

|
| Exo Dimer | Endo Dimer |
| Isomer | 3D representation | Total Energy/ kcal mol-1 |
|---|---|---|
| Exo Dimer | 31.77
| |
| Endo Dimer | 33.92
|
The computational results show that the exo dimer would be the thermodynamic product and is 2.15 kcal mol-1more stable than the endo dimer. However since we know that the endo dimer is formed, the reaction must be kinetically controlled and the transition state for the formation of the endo dimer must be lower in energy than that of the exo dimer.
The partial hydrogenation of the endo dimer can occur at two different places to give two different products A and B shown below. The relative contributions from the stretching, bending, stretch-bend, torsion, van der Waals and hydrogen bonding energy terms are shown together with the combined total energy.
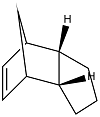 |

|
| Product A | Product B |
| Isomer | 3D representation | Stretching/ kcal mol-1 | Bending/ kcal mol-1 | Stretch-bend/ kcal mol-1 | Torsion/ kcal mol-1 | van der Waals/ kcal mol-1 | Hydrogen bonding/ kcal mol-1 | Total Energy/ kcal mol-1 |
|---|---|---|---|---|---|---|---|---|
| Product A | 1.28 |
19.80 |
-0.84 |
10.87 |
4.42 |
0.16 |
35.70
| |
| Product B | 1.10 |
14.55 |
-0.55 |
12.50 |
3.41 |
0.14 |
31.16
|
The data shows that product B is 4.54 kcal mol-1 more stable than product A. This is mainly due to the particularly high torsional strain term in product A.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
The reaction of an optically active derivative of prolinol with methyl magnesium iodide proceeds as shown in the reaction scheme below. The stereochemistry of the product is absolute as shown in the product.
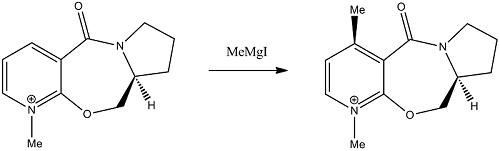
The regio- and stereoselectivity of the reaction can be rationalised through the involvement of the carbonyl of the amide group. In the first step of the mechanism, the oxygen of the amide group coordinates to the magnesium of the Grignard reagent and acts as a tether.[1] The delivery of the methyl group is therefore directed to the 4-position of the pyridinium ring through the formation of an intermediate containing a six-membered ring. This explains the regioselectivity of the reaction. In order to explain the stereoselectivity we must first consider the geometry of the prolinol derivative reactant.
The geometry of the prolinol derivative was optimised using MM2. This model was used to calculate the dihedral angle O-C-C-C, shown visually in Figure ??? below. However due to a bug in the ChemBio3D program which affects molecules containing an N+ atom, a second optimisation using the MOPAC PM6 molecular orbital method was used in order to give a more accurate geometry.
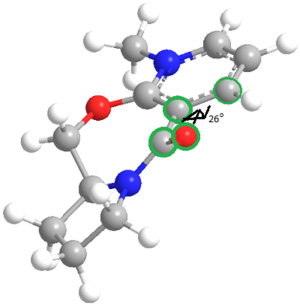
| Method | Total Energy/kcal mol1 | Dihedral Angle/o |
|---|---|---|
| MM2 | 43.11 |
10
|
| MMFF94 | 56.46 |
15
|
| MOPAC | Heat of Formation = 93.89 kcal mol-1 |
26
|
The dihedral angle found when optimised using the MM2 method and using the MOPAC method differed significantly.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedasdfef
Reaction 1
MM2
Stretch: 2.0526
Bend: 14.2422
Stretch-Bend: 0.1313
Torsion: 5.0869
Non-1,4 VDW: -0.5290
1,4 VDW: 16.5432
Charge/Dipole: 9.5943
Dipole/Dipole: -4.0083
Total Energy: 43.1132 kcal/mol
Dihedral angle 10
MMFF94
Total energy 56.4613 kcal/mol
Dihedral angle 15
MOPAC
Heat of Formation = 93.89241
Dihedral angle 26
Reaction 2
MM2
Stretch: 6.0280
Bend: 18.5770
Stretch-Bend: 0.5342
Torsion: 18.7564
Non-1,4 VDW: 6.9163
1,4 VDW: 29.7034
Charge/Dipole: 8.3605
Dipole/Dipole: -4.9168
Total Energy: 83.9590 kcal/mol
Dihedral angle 25
MMFF94
Total energy 113.153 kcal/mol
Dihedral angle 40
MOPAC
Heat of Formation 157.44527
Dihedral angle 45
