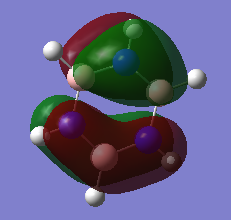KG971003
EX3 project
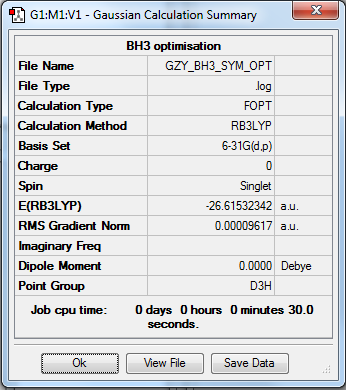
BH3
Optimisation
• Method:RB3LYP
• Basis set:6-31G
•E=-26.61532 a.u.
Item Value Threshold Converged? Maximum Force 0.000192 0.000450 YES RMS Force 0.000126 0.000300 YES Maximum Displacement 0.000763 0.001800 YES RMS Displacement 0.000500 0.001200 YES
•link to frequency .log file: GZY_BH3_FREQ.LOG
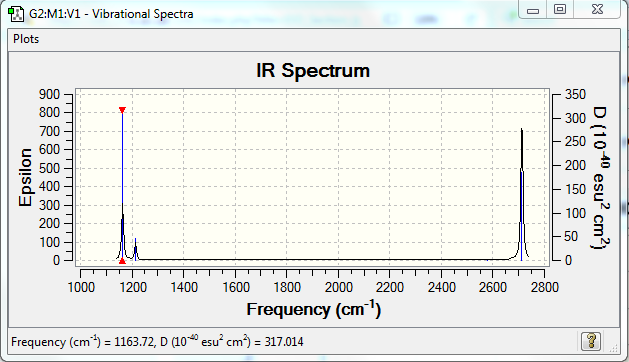
Low frequencies --- -0.2263 -0.1037 -0.0054 47.9770 49.0378 49.0383 Low frequencies --- 1163.7209 1213.6704 1213.6731
BH3 |
| wavenumber (cm-1) | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 92 | A1 | yes | out-of-plane bend |
| 1213 | 14 | E | slight | bend |
| 1213 | 14 | E | slight | bend |
| 2579 | 0 | A1 | no | symmetric stretch |
| 2712 | 126 | E | yes | asymmetric stretch |
| 2712 | 126 | E | yes | asymmetric stretch |
•There are only 3 peaks visible in the spectrum, which is fewer than 6 peaks derived from 3N-6 rule. The reason is that some of the vibrations are not IR active, such as the symmetric stretch. In addition to that, there are two vibrations with the same frequency, this lead to the overlap of two peaks to give a larger one.
Ng611 (talk) 18:07, 30 May 2018 (BST) Which vibrations are inactive, and which ones are degenerate? Be more specific. Otherwise, good!
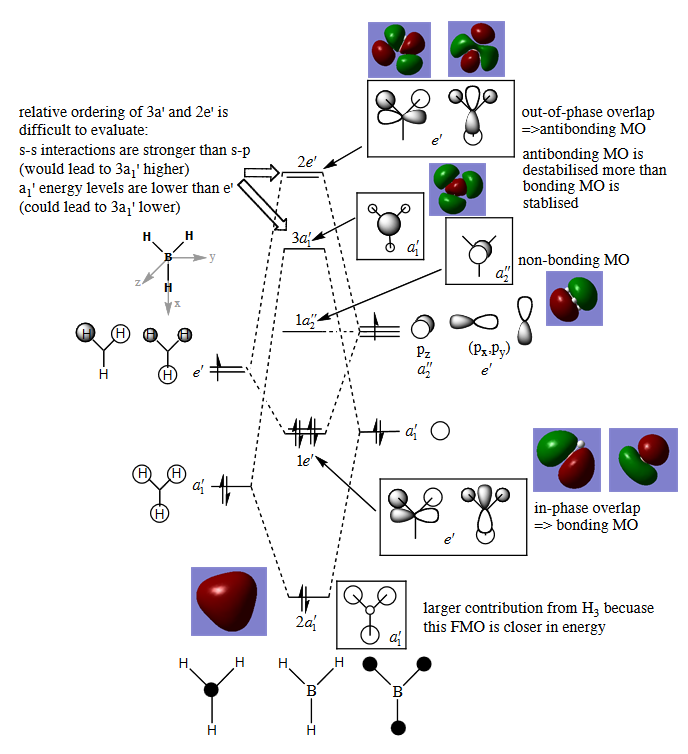
MO diagram for BH3
Lecture 4 Tutorial Problem Model Answers from Dr. Patricia Hunt
•There are not many significant differences between the real and LCAO MOs. This shows that the qualitative MO theory is accurate and useful.
Ng611 (talk) 18:08, 30 May 2018 (BST) Are there any differences?
Association energy
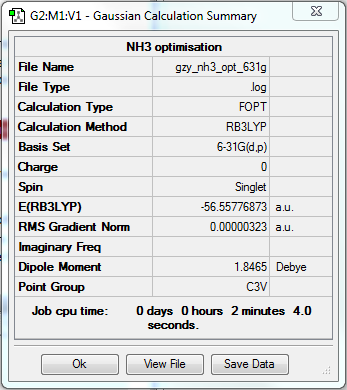
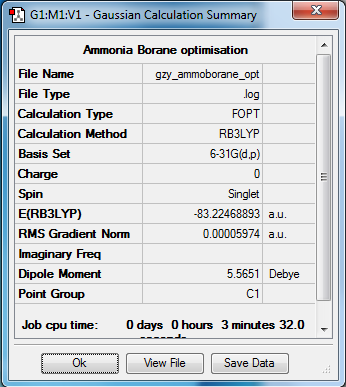
NH3 optimisation
NH3BH3 optimisation
Ng611 (talk) 18:09, 30 May 2018 (BST) You're missing the remaining information (log files, etc.) for these calculations.
ΔE = E(NH3BH3) - [E(NH3)+E(BH3)] = -0.05159678 a.u.= -135 kJ/mol
•It is a sensible value. Normal covalent single bond strength is between 200-500 kJ/mol. This is a weak dative bond because it is much weaker than C-C bond which is about 350 kJ/mol.
Ng611 (talk) 18:09, 30 May 2018 (BST) Remember to cite a literature value for your bond enthalpy (ideally from a paper source).
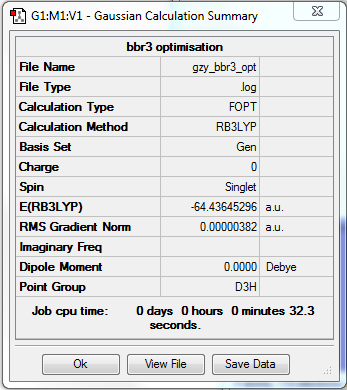
BBr3
Optimisation
•Method:RB3LYP
•Basis set:Gen
Ng611 (talk) 18:10, 30 May 2018 (BST) Be careful here. "Gen" is not a valid descriptor for this basis set. You actually mean something like LANL2DZ/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000023 0.001200 YES
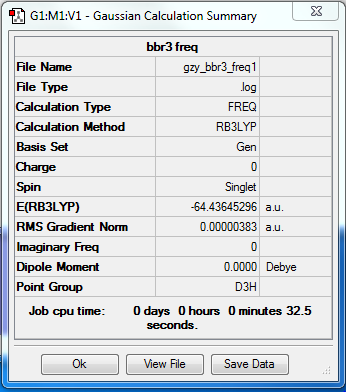
Frequency analysis
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000018 0.001200 YES
Low frequencies --- -0.0137 -0.0064 -0.0046 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
Benzene and Borazine
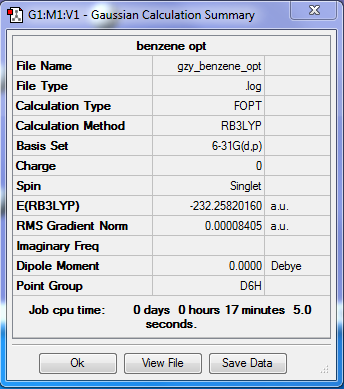
Benzene optimisation
•Method:RB3LYP
•Basis set:6-31G
Item Value Threshold Converged? Maximum Force 0.000194 0.000450 YES RMS Force 0.000077 0.000300 YES Maximum Displacement 0.000824 0.001800 YES RMS Displacement 0.000289 0.001200 YES
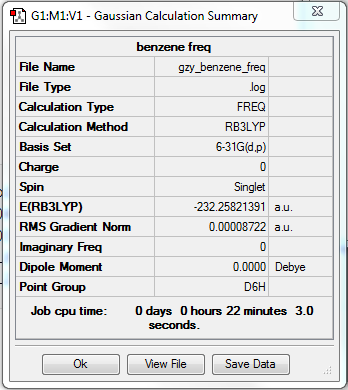
Benzene frequency analysis
•Method:RB3LYP
•Basis set:6-31G
Item Value Threshold Converged? Maximum Force 0.000198 0.000450 YES RMS Force 0.000087 0.000300 YES Maximum Displacement 0.000757 0.001800 YES RMS Displacement 0.000321 0.001200 YES
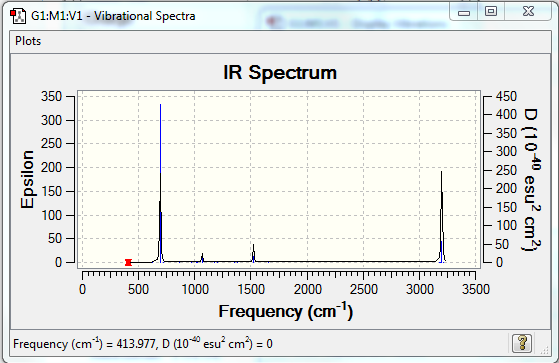
Low frequencies --- -2.1456 -2.1456 -0.0088 -0.0043 -0.0042 10.4835 Low frequencies --- 413.9768 413.9768 621.1390
•IR spectrum---all positive
•Jmol image of Benzene
Benzene |
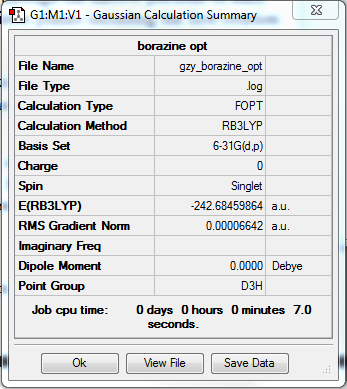
Borazine optomisation
•Method:RB3LYP
•Basis set:6-31G
Item Value Threshold Converged? Maximum Force 0.000068 0.000450 YES RMS Force 0.000037 0.000300 YES Maximum Displacement 0.000409 0.001800 YES RMS Displacement 0.000136 0.001200 YES
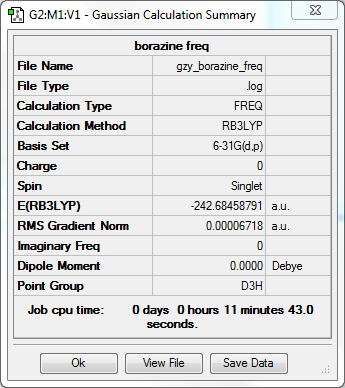
Borazine frequency analysis
•Method:RB3LYP
•Basis set:6-31G
Item Value Threshold Converged? Maximum Force 0.000201 0.000450 YES RMS Force 0.000067 0.000300 YES Maximum Displacement 0.000448 0.001800 YES RMS Displacement 0.000179 0.001200 YES
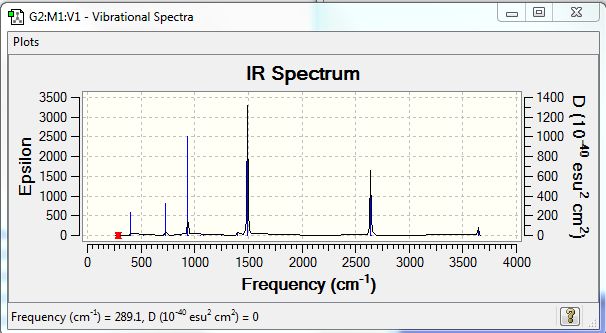
Low frequencies --- -11.1371 -10.9083 -10.7025 -0.0104 -0.0093 0.0939 Low frequencies --- 289.1003 289.1090 403.9346
•IR spectrum-- all positive
•Jmol image of borazine
Benzene |
Charge analysis
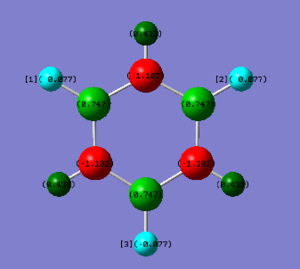
Charge distribution of Benzene(left) and Borazine(right)
• Charge analysis benzene
| Benzene | Electronegativity | Charge / a.u. |
| C | 2.5 | -0.239 |
| H | 2.1 | +0.239 |
Ng611 (talk) 18:12, 30 May 2018 (BST) Remember to use the same colour scale for both molecules.
• Charge analysis borazine
| Borazine | Electronegativity | Charge / a.u. |
| N | 3.0 | -1.102 |
| B | 2.0 | +0.747 |
| H-N | 2.1 | +0.432 |
| H-B | 2.1 | -0.077 |
-The charge distribution is dependent on the electronegativity of atoms in Benzene and Borazine.
-In Benzene, C is more electronegative than H, resulting in a greater electron density around C. This leads to the positive charge on H and negative charge on C.
-In Borazine, N is the most electronegative atom, therefore, the electron density around N is the greatest. This is consistent with the most negative charge on N which is -1.102 au. As H has a slight larger electronegativity than B, H atom connected to B have a slightly negative charge which means B-H bond is less polarised, while those connected to N is positively charged which lead to a more polarized N-H bond.
Ng611 (talk) 18:13, 30 May 2018 (BST) Good discussion of the effects of electronegativity on the overall charge distribution but more discussion is needed. What do the partial charges sum to, and is there any difference in partial charge for atoms related by symmetry?
MOs comparison
Ng611 (talk) 18:15, 30 May 2018 (BST) Well done for comparing the correct MOs by shape and not energetic ordering (which is not necessarily reliable). I would include a brief discussion of the overall symmetry and perhaps a more detailed discussion of the differences between the molecules to improve this section further. Perhaps also consider dicussing the constituent AOs that form the MOs and the overall symmetry of the MO.
Aromaticity
At first, aromaticity is related to Benzene. Aromaticity is usually used to describe a cyclic, planar molecule with the number of resonace bonds obeying Hackle's law and is more stable than other geometry with the same set of atoms. The stability is due to the delocalised system of pi orbitals as a result of pz orbital overlapping as shown in Benzene MO diagram above. In Borazine, the overlap is between hetroatoms. ie. nitrogen and Boron. The difference in energy and electronegativity can result in distortion of the pi system.
However, it is now well known that aromaticity can also be applied on non-planar molecules because the planrity can be destroyed easily, eg, benzene adopts a chair conformation in crystalline state at 20K. A good way to investigate the pi system is the Bader quantum theory atons in molecules.
Ng611 (talk) 18:20, 30 May 2018 (BST) How is aromaticity maintained upon the destruction of planarity? Can you find aromaticity in other non-planar molecules? What other modern perspectives on aromaticity are there. What you have written is correct, but overall more discussion is needed.
Ng611 (talk) 18:20, 30 May 2018 (BST) Some good aspects of this report. However, the level of detail in your discussion (especially in the second section) should be much greater. You have performed your calculations correctly and obtained good results, but you need to analyse them in greater depth.