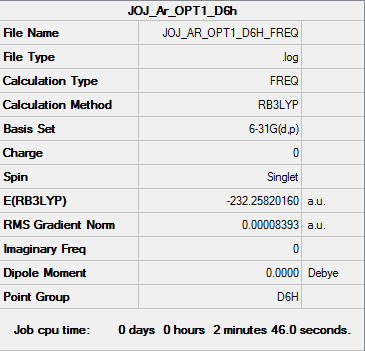
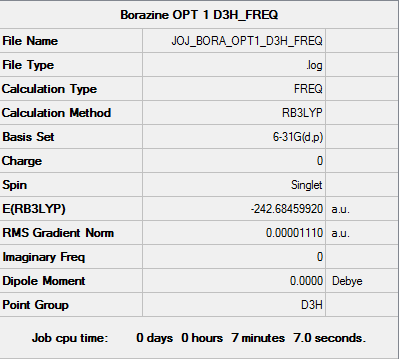
Jojaromatic
Project: Aromaticity
Charge Distribution
Both borazine and benzene are neutral molecules. Thus, it is expected that the sum of all the partial charges is equal to zero. ***This is indeed the case.
The partial charges on the atoms arise from the polarisation of the covalent bonds due to the differences in electronegativity of the atoms of said bonds. The greater the difference in electronegativity between the two atoms in a bond, the greater the polarisation / ionic character of the bond. This results it greater partial charges residing on each atom.
In benzene, C - C and C - H bonds are present. According to the Pauling electronegativity scale, C has an electronegativity (χ) of 2.54 while χH = 2.3. The C - H bonds are only slightly polar due to the similarity in these values; small negative and small positive charges reside on the C and H atoms respectively. The C - C bonds are not polarised.
In borazine, B - N, N - H and B- H bonds are present. The values of χ are as follows:
- χB = 2.05
- χH = 2.30
- χN = 3.07
From the above values, a number of predictions may be made. The difference in electronegativity (Δχ) between C and H is less than that between B and H and N and H. Thus, it is predicted that the N - H and B - H bonds will be more polarised than the C - H bonds. Additionally, while the ring atoms in benzene are all identical, the B - N bonds in borazine are highly polarised: as each B atom is bonded to two N atoms of much greater electronegativity (and vice versa), a high positive/negative partial charge is expected to reside on the B/N atoms.
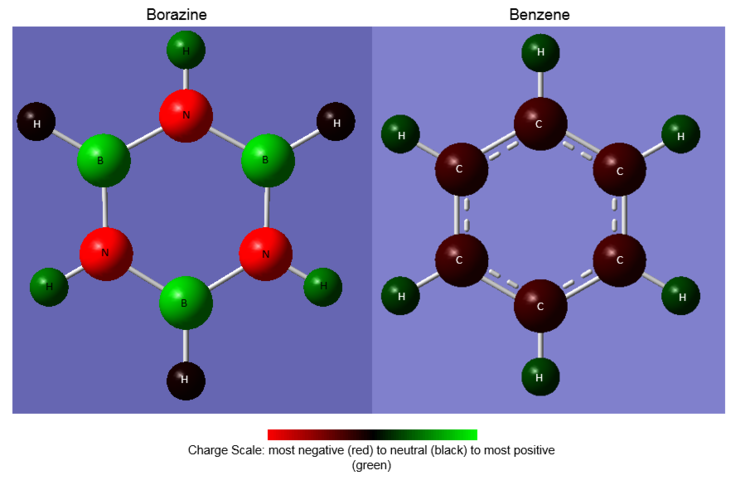
Figure ___ displays the NBO charge distributions for both molecules with a charge scale for comparison. The numerical values of the partial charges are listed in Table 2
| Molecule | Atom | Bond(s) | Partial Charge / e |
|---|---|---|---|
| Benzene | C | C - C / C - H | -0.239 |
| H | C - H | 0.239 | |
| Borazine | N | B - N - B / N - H | -1.102 |
| B | N - B - N / B - H | 0.747 | |
| H | B - H | -0.077 | |
| H | N - H | 0.432 |
Indeed it has been found that the bonds in borazine are more polarised than those in benzene; greater partial charges (measured in units of e, atomic unit of charge / magnitude of electron charge / elementary charge) reside on the ring atoms in borazine than on the ring atoms in benzene. In other words, there is a much greater delocalisation of charge around the ring atoms in benzene than in borazine.
Aromaticity
> Resonance structures in benzene equal energy?
> Mesomeric resonance structures of borazine are possible but contribute very little to structure (due to high energy structures where - charge resides on B atom and + atom on N atom)
> Borazine obeys Huckel's Rules
> All B - N lengths in borazine are equal
> Yet Borazine has high energy! Redefinition of aromaticity is needed
This result provides interesting insight into the concept of aromaticity given that benzene (the prototypical aromatic structure) is isoelectronic with borazine. The stabilisation may be attributed to the aromatic resonance stabilisation of benzene. The conclusion of the charge analysis is such that the origin of this aromatic stabilisation is the delocalisation of charge around the benzene ring.
Low frequencies --- -16.9682 -14.6636 -14.6636 -0.0055 -0.0055 0.0001 Low frequencies --- 414.1239 414.1239 620.9400
Fig ____ The vibrations obtained for benzene
Link to benzene frequency analysis log file
Low frequencies --- -4.8064 -4.7828 -4.0590 -0.0030 0.0035 0.0050 Low frequencies --- 289.6877 289.6884 404.4509
Fig ____ The vibrations obtained for borazine
Link to borazine frequency analysis log file