Jo416i
Inorganic Computational Lab
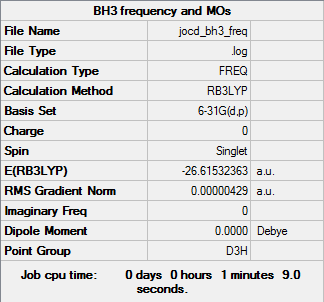
BH3
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000009 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000034 0.001800 YES RMS Displacement 0.000017 0.001200 YES
Frequency file: bh3_frequency.log
Low frequencies --- -2.2126 -1.0751 -0.0055 2.2359 10.2633 10.3194 Low frequencies --- 1162.9860 1213.1757 1213.1784
optimised BH molecule |
Vibrational spectrum for BH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 93 | A2 | yes | out-of-plane bend |
| 1213 | 14 | E' | slight | in-plane-bend |
| 1213 | 14 | E' | slight | In-plane-bend |
| 2582 | 0 | A1' | no | symmetric stretch |
| 2715 | 126 | E' | yes | asymmetric stretch |
| 2715 | 126 | E' | yes | asymmetric stretch |
Only three peaks are seen in the IR spectrum, despite there being 6 vibrations present. 1 of these vibrations (2582 cm-1) is a symmetric stretch, there is therefore no change in overall dipole moment and thus is not seen in the spectra. There are also two pairs of degenerate vibrations which overlap and therefore you only see 1 peak for each pair. This results in 3 peaks seen in the above spectra.
BH3 Molecular Orbital Diagram

Comparisons of the real molecular orbitals versus the molecular orbitals obtained through LCAO shows the power of molecular orbital theory in predicting real, filled orbitals. However, the accuracy and usefulness of MO theory breaks down for the unoccupied orbitals higher up the diagram. There are significant differences in the contributions of the fragment orbitals predicted vs in the 'real' MOs.
Smf115 (talk) 19:20, 23 May 2018 (BST)Good inclusion of the MOs and the comparison picks up on both the similarities and the differences of the calculated MOs and the LCAO qualitative approach.
Association energies: Ammonia-Borane
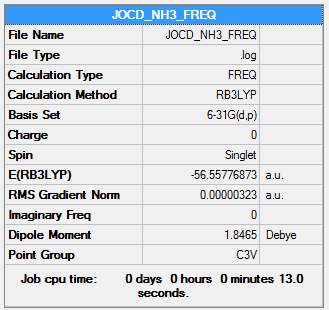
NH3
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000014 0.001800 YES RMS Displacement 0.000009 0.001200 YES
Frequency file: NH3_frequency.log
Low frequencies --- -0.0128 -0.0010 0.0014 7.1032 8.1046 8.1049 Low frequencies --- 1089.3834 1693.9368 1693.9368
optimised NH molecule |
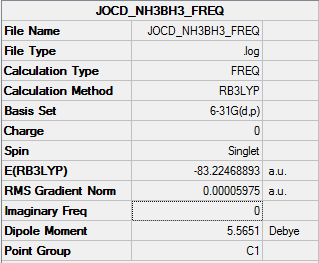
NH3BH3
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000122 0.000450 YES RMS Force 0.000058 0.000300 YES Maximum Displacement 0.000582 0.001800 YES RMS Displacement 0.000320 0.001200 YES
Frequency file: NH3BH3_frequency.log
Low frequencies --- -0.0014 -0.0011 -0.0009 16.8481 17.4133 37.2932 Low frequencies --- 265.8219 632.2116 639.3277
optimised NHbH molecule |
Determining the energy of the dative bond
E(NH3)=-56.5578 a.u.
E(BH3)=-26.6153 a.u.
E(NH3BH3)=-83.2247 a.u.
ΔE=E(NH3BH3)-[E(BH3)+E(NH3)]
ΔE=-0.0516 a.u.
ΔE=-140 kJ/mol (rounded to the nearest 10 kJ/mol)
(lit[2]: 130 kJ/mol)
The dative B-N bond is very weak compared to its covalent equivalents. For instance the H3C-CH3 also shares one electron pair in valence bond theory, however it has a much higher bond strength of roughly 380 KJ/mol[3].
Smf115 (talk) 19:19, 23 May 2018 (BST)Well referenced answer and correct calculation. Good consideration of the accuracy for the reported energy values, however, although only really accurate to the nearest 10 kJ/mol the energy is reported to the nearest 1 kJ/mol.
BBr3
B3LYP/6-31G(d,p)LANL2DZ
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000023 0.001200 YES
Frequency file: BBr3_frequency.log
Low frequencies --- -0.0137 -0.0064 -0.0046 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
optimised BBr molecule |
Ionic Liquids
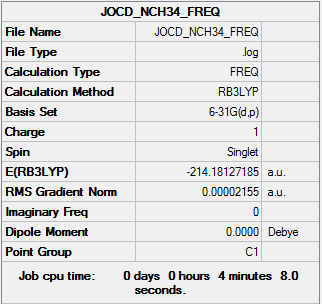
[N(CH3)3]+
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000076 0.000450 YES RMS Force 0.000017 0.000300 YES Maximum Displacement 0.001121 0.001800 YES RMS Displacement 0.000321 0.001200 YES
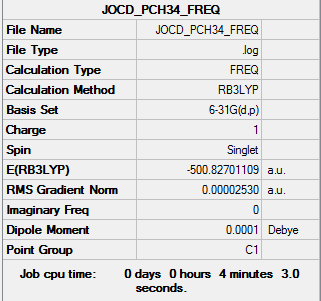
Frequency file: NCH34_frequency.log
Low frequencies --- -9.9670 -0.0011 -0.0007 0.0000 3.4930 8.1265 Low frequencies --- 182.5341 288.5524 288.7594
optimised BBr molecule |
Smf115 (talk) 19:19, 23 May 2018 (BST)Great structure information and inclusion of the charges on the compounds.
[P(CH3)3]+
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000146 0.000450 YES RMS Force 0.000032 0.000300 YES Maximum Displacement 0.001509 0.001800 YES RMS Displacement 0.000498 0.001200 YES
Frequency file: NCH34_frequency.log
Low frequencies --- -4.5064 0.0014 0.0029 0.0029 10.2391 13.4026 Low frequencies --- 157.0126 192.4654 192.6170
optimised BBr molecule |
NBO Charge Analysis
![NBO charge analysis of [N(CH3)3]+ (left) vs. [P(CH3)3]+]](/images/thumb/c/cc/JOCD_COMB_CHARGE.png/800px-JOCD_COMB_CHARGE.png)
[N(CH3)3]+
Atom: Charge
N: -0.295,
C: -0.483,
H: 0.269,
[P(CH3)3]+
Atom: Charge
P: 1.667,
C: -1.060,
H: 0.298,
The charge analysis shows marked differences in the charge distribution between the two structures. In [P(CH3)3]+ , the positive charge is localised significantly on the phosphorous centre. In comparison the nitrogen centre in [N(CH3)3]+ holds a slight negative charge.
The traditional description of [N(CH3)3]+ would suggest a localized, negative charge centered on the nitrogen. As the above analysis shows, this model fails to explain the true nature of the positive charge in the compound. In this case, the positive charge is more delocalised across the entire molecule. Given their natural polarities, nitrogen would be expected to hold a more significant negative partial charge than carbon. This is the behaviour seen in [P(CH3)3]+.
The difference in behaviour is most easily explained by consideration of electronegativities. The difference in electronegativity between P and C is much greater than between N and C, as such the electron density is 'pulled' more strongly towards the P than the N.
This charge delocalisation is pivotal in the low melting point of the ionic liquids (important for their use as solvents). The delocalised charge in the nitrogen compound promotes poor coordination with the anions and thus a lower melting point for the salt. This charge delocalisation (and thus melting point) can be increased by changing the R groups on the cation, pyridinium ions are examples of groups which result in significant charge delocalisation.
The phosphorous compound, with its more localized charge, likely has a much higher melting point. For something to be useful as an ionic solvent it is ideally needs to have a melting point of <100 °C.
Smf115 (talk) 23:07, 22 May 2018 (BST)Good to see the charge distribution set to the same range across both molecule. The use of electronegativities to explain the charge differences is good however, there are a few errors such as electron density wouldn't be pulled to the P as C is actually more electronegative. The analysis could have been improved by mentioning other factors such as symmetry and the traditional depiction of the positive charge located on the N was not explained. Nice extra information about ionic liquids brought in at the end though.
Molecular Orbital Analysis
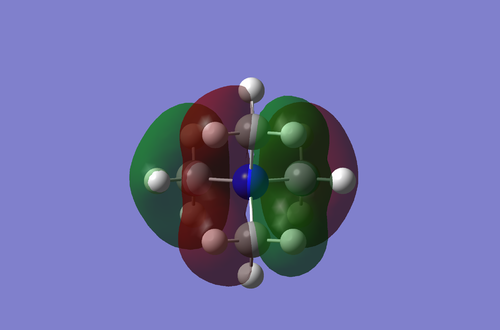
The MO above is strongly C-H bonding, with good S-like overlap on the methyl fragments. It is relatively C-N non-bonding with the no nodes sitting directly on the bond.
This MO shows both sigma like and pi like bonding character. The p-orbital like fragments at the front show sigma like C-H bonding character as well as pi like C-N bonding character. The fragments behind the plane have p-like orbitals pointing directly towards the nitrogen center. This results in good overlap with the p-orbital on the nitrogen and results in relatively strong C-N sigma bonding character.
MO19 is a completely bonding orbital. This can be seen quickly by all the nodal planes sitting directly on atoms and none on bonds. There is strong overlap between the 'p-like' fragment orbitals and the pz orbital on the nitrogen center. There is also strong C-H bonding character from the overlap of the of the carbon p orbital with the the s orbitals on the hydrogens.
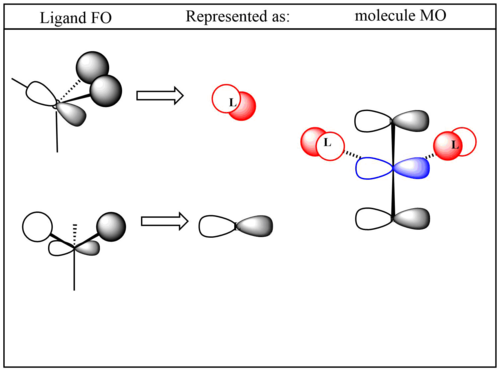
Smf115 (talk) 19:30, 23 May 2018 (BST)Good range of MOs shown and the FOs and LCAOs are largely correct. Consideration should be given to the picture used for the MO, it can be hard to show them well with a single screenshot but a better image (or two) could have been chosen for MO 13. The FOs for MO 13 could also be improved with the red FO not quite correct and could probably be drawn a bit clearer.
Smf115 (talk) 19:30, 23 May 2018 (BST)Overall a good report with an excellent first section.
- ↑ Hunt, T. (2018) Molecular Orbitals in Inorganic Chemistry. [Lecture] Imperial College London, 2018
- ↑ HAALAND, A. (1989). ChemInform Abstract: Covalent and Dative Bonds to Main Group Metals - A Useful Distinction. ChemInform, 20(43).
- ↑ HAALAND, A. (1989). ChemInform Abstract: Covalent and Dative Bonds to Main Group Metals - A Useful Distinction. ChemInform, 20(43).