Jega2603
Introduction
GaussView was used to optimise the molecule. Images and information like atomic charges, bond angles and vibrations of molecules were obtained using GaussView too.The molecules analysed were ammonia,hydrogen,nitrogen,hydrochoric acid and chlorine. Molecular orbitals of hydrochloric acid and chlorine were analysed too.
Ammonia (NH3)
test molecule |
Summary
Calculation method: RB3LYP
Basis Set: 6-31G(d.p)
Calculation type: Frequency
Final energy E(RB3LYP):-56.55776873 a.u.
RMS gradient:0.00000485 a.u.
Point group: C3V
Spin: Singlet
N-H bond length: 1.01798Å
H-N-H bond angle:105.741°
'Item' table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES Predicted change in Energy=-5.986283D-10 Optimization completed. -- Stationary point found.
Completed Ammonia optimisation *.log file
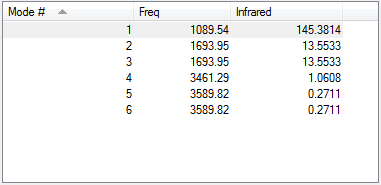
Vibrations of Ammonia
1. 6 modes of vibrations are expected for ammonia molecule. There are a total of 4 atoms in the molecule,so the number of vibrations = 3(4)-6=6.
2. The vibrations with the same frequency are degenerate. So, vibrational modes 2 and 3, and 5 and 6 are degenerate.
3. Modes 4,5 and 6 are are 'bond stretch' vibrations while modes 1,2 and 3 are bending vibrations.Bending vibrations have higher frequency.
4. Vibration modes 4 is highly symmetric.
5. Mode 1 is the umbrella mode.
6. 2 different bands can be seen. Modes of vibrations having the three highest frequencies have larger changes in dipole moment compared to the remaining three.Two of the frequencies are degenerate,so,two bands are visible. The lowest three frequencies are not visible because the change in dipole moment is so small that they form very small peaks.
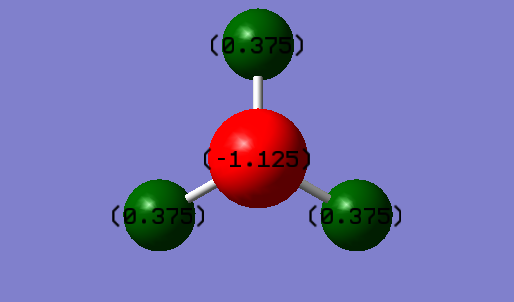
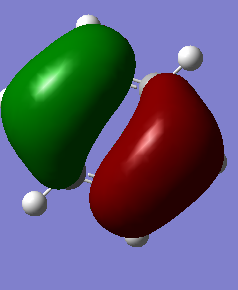
Atomic Charges of Ammonia
The atomic charge on nitrogen atom is -1.125 while each of the hydrogen atoms carry a positive charge of 0.375.
I would expect a negative charge on the nitrogen atom as it is more electronegative than the hydrogen atoms. Thus, it pulls the electron density shared between itself and the hydrogen atoms towards itself leaving the hydrogen atoms with lesser electron density. As electrons are negatively-charged, this increases the negative charge on nitrogen atom. Hydrogen atoms which have lost some electron density have slight build-up of positive charge.
Hydrogen(H2)
test molecule |
Summary
Calculation method: RB3LYP
Basis Set: 6-31G(d.p)
Calculation type: Frequency
Final energy E(RB3LYP):-1.17853930 a.u.
RMS gradient:0.00012170 a.u.
Point group: D∞h
Spin: Singlet
Bond distance: 0.74309Å
Bond angle:180°
'Item' table
Item Value Threshold Converged? Maximum Force 0.000211 0.000450 YES RMS Force 0.000211 0.000300 YES Maximum Displacement 0.000278 0.001800 YES RMS Displacement 0.000393 0.001200 YES Predicted change in Energy=-5.852867D-08 Optimization completed. -- Stationary point found.
Completed Hydrogen optimisation *.log file
Vibrations of Hydrogen
There will be no peak observed as there is no change in dipole moment. The peak intensity of 0.0000 proves this.
Atomic Charges of Hydrogen
They have zero charge because there is no difference in electronegativity between the two atoms.
Nitrogen(N2)
test molecule |
Summary
Calculation method: RB3LYP
Basis Set: 6-31G(d.p)
Calculation type: Frequency
Final energy E(RB3LYP): -109.52412868 a.u.
RMS gradient: 0.00000365 a.u.
Point group: D∞h
Spin: Singlet
Bond distance: 1.10550 Å
Bond angle:180°
'Item' table
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000002 0.001800 YES RMS Displacement 0.000003 0.001200 YES Predicted change in Energy=-1.248810D-11 Optimization completed. -- Stationary point found.
Completed Nitrogen optimisation *.log file
Vibrations of Nitrogen
There will be no peak observed as there is no change in dipole moment. The peak intensity of 0.0000 proves this.
Atomic Charges of Nitrogen
They have zero charge because there is no difference in electronegativity between the two atoms.
Haber-Bosch calculation
Enthalpy calculation
E(NH3)= -56.55776873 a.u.
2*E(NH3)= -113.1155375 a.u.
E(N2)=-109.52412868 a.u.
E(H2)=-1.17853930 a.u.
3*E(H2)=-3.5356179 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579092 a.u.
ΔE=-146.4790605 kJ/mol
As ΔE is less than zero, the process is exothermic.This shows that ammonia product is more stable as the process of conversion of the gaseous reactants to ammonia releases energy.
Comparison with literature value
The literature value for this reaction is -92.4 kJ/mol.[1] The enthalpy obtained after optimisation of molecules is larger than the literature value.This might due to experimental conditions like the use of catalyst and temperature or pressure difference.It might be due to solvation effect too.
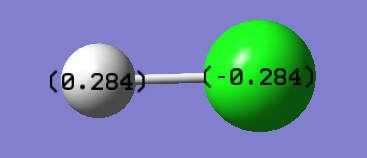
Hydrochloric Acid(HCl)
test molecule |
Summary
Calculation method: RB3LYP
Basis Set: 6-31G(d.p)
Calculation type: Frequency
Final energy E(RB3LYP): -460.80077876 a.u.
RMS gradient: 0.00000004 a.u.
Point group: C∞V [2]
Spin: Singlet
Bond distance: 1.28613 Å
Bond angle:180°
'Item' table
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES Predicted change in Energy=-9.318527D-15 Optimization completed. -- Stationary point found.
Completed HCl.log file
Vibrations of HCl
Atomic Charges of HCl
Chlorine atom is more electronegative than the hydrogen atom. Thus, it pulls the electron density shared between itself and the hydrogen atom towards itself leaving the hydrogen atom with lesser electron density. As electrons are negatively-charged, this increases the negative charge on chlorine atom. Hydrogen atom which has lost some electron density has slight build-up of positive charge.
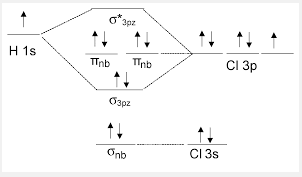
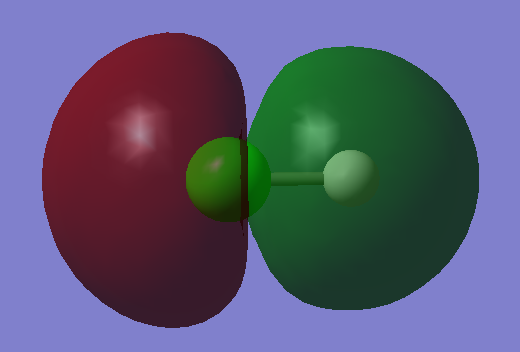
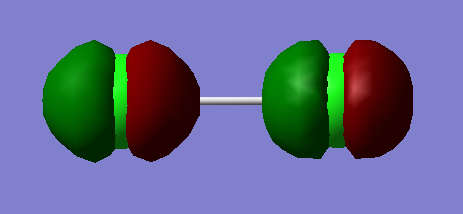
Molecular Orbital Diagram of HCl
Molecular Orbitals
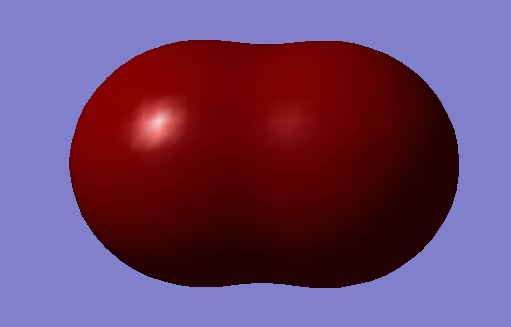
1s orbital of chlorine contributes to this molecular orbital. The 1s orbital of chlorine is too low in energy to overlap with the only 1s orbital of hydrogen. It is a non-bonding molecular orbital which is the lowest in energy among all the molecular orbitals. It is occupied with electrons from 1s orbital of chlorine. It has no effect on bonding.
2p orbital of chlorine contributes to this molecular orbital.The 2p orbital of chlorine is not high enough in energy to overlap with the 1s orbital of hydrogen. It is a non-bonding molecular orbital which is quite deep in energy.The molecular orbital is occupied by respective 2p electrons of chlorine and it has no effect on bonding.
3s orbital of chlorine contributes to this molecular orbital.The 3s orbital of chlorine is not high enough in energy to overlap with the 1s orbital of hydrogen It is a non-bonding molecular orbital which is quite high in energy. The molecular orbital is occupied with electrons from 3s orbital of chlorine and it has no effect on bonding.
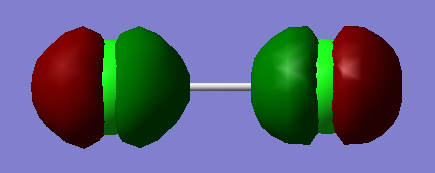
1s orbital of hydrogen and 3p orbital of chlorine contribute to this molecular orbital.It is a bonding molecular orbital which is quite high in energy.The molecular orbital is occupied. It forms the sigma bond between hydrogen and chlorine.
3p orbital of chlorine contribute to this molecular orbital. It is a non-bonding molecular orbital which is quite high in energy.The molecular orbital is occupied. It is the highest occupied molecular orbital(HOMO)of HCl.
1s orbital of hydrogen and 3p orbital of chlorine contribute to this molecular orbital.It is an antibonding molecular orbital which is quite high in energy.The molecular orbital is not occupied and it has no effect on bonding. This orbital is the lowest unoccupied molecular orbital (LUMO) of HCl.
Chlorine(Cl2)
test molecule |
Summary
Calculation method: RB3LYP
Basis Set: 6-31G(d.p)
Calculation type: Frequency
Final energy E(RB3LYP): -920.34987886 a.u.
RMS gradient:0.00000661 a.u.
Point group: D∞h [2]
Spin: Singlet
Bond distance: 2.04164 Å
Bond angle:180°
'Item' table
Item Value Threshold Converged? Maximum Force 0.000011 0.000450 YES RMS Force 0.000011 0.000300 YES Maximum Displacement 0.000032 0.001800 YES RMS Displacement 0.000045 0.001200 YES Predicted change in Energy=-3.658847D-10 Optimization completed. -- Stationary point found.
Completed Chlorine optimisation *.log file
Vibrations of Chlorine
There will be no peak observed as there is no change in dipole moment. The peak intensity of 0.0000 proves this.
Atomic Charges of Chlorine
They have zero charge because there is no difference in electronegativity between the two atoms.
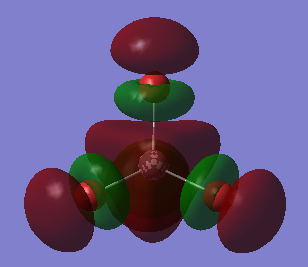
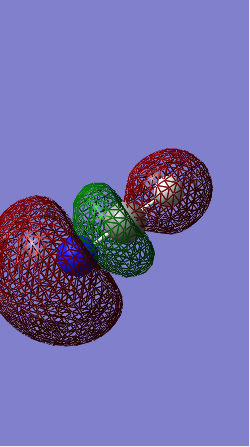
Molecular orbitals
Two 1s orbitals of chlorine contribute to this molecular orbital.It is a non-bonding molecular orbital which is the deepest in energy among all the molecular orbitals. It is occupied and has no effect on bonding.
This is an antibonding molecular orbital. 2s orbitals contribute to this molecular orbital. They are out of phase of each other. It is quite deep in energy. It is occupied and has effect on bonding.However, the existence of corresponding occupied bonding orbital cancels out the effect on bonding.
This is an antibonding orbital too where the orbitals which combine here are the 2p orbitals of the chlorine. This orbital is higher in energy than the antibonding orbital formed from 2s orbitals.The orbital is occupied and has effect on bonding.However, the existence of corresponding occupied bonding orbital cancels out the effect on bonding.
This is a sigma bonding orbital obtained from the linear combination of two 2p orbitals which overlaps in a linear manner.It is quite high in energy. It is occupied and has effect on bonding.However, the existence of corresponding occupied antibonding orbital cancels out the effect on bonding.
This is a sigma bonding orbital which results from the in-phase combination of two 3s orbitals. It is quite high in energy. It is occupied and has effect on bonding.However, the existence of corresponding occupied antibonding orbital cancels out the effect on bonding.
Reference
- ↑ http://chemwiki.ucdavis.edu/Core/Physical_Chemistry/Equilibria/Case_Studies/Haber_Process(4th March 2015)
- ↑ 2.0 2.1 http://cccbdb.nist.gov/pglistx.asp.(4th March 2015)
- ↑ http://chemistry.stackexchange.com/questions/15205/energetic-placement-of-atomic-orbitals-in-the-hcl-molecular-orbital-diagram (4th March 2015)