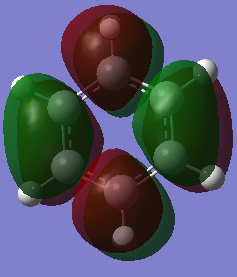JakePetersInorganicWikiY2
Inorganic Lab Wiki
EX3 Section
BH3
B3YLP/6-63G level
BH3Summary Table
Item Value Threshold Converged?
Maximum Force 0.000009 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000034 0.001800 YES
RMS Displacement 0.000017 0.001200 YES
Frequency analysis log file File:JP BH3 FREQ.log
low frequencies
Low frequencies --- -2.3742 -1.0821 -0.0053 2.0837 10.1950 10.2522 Low frequencies --- 1162.9856 1213.1754 1213.1781
BH3 |
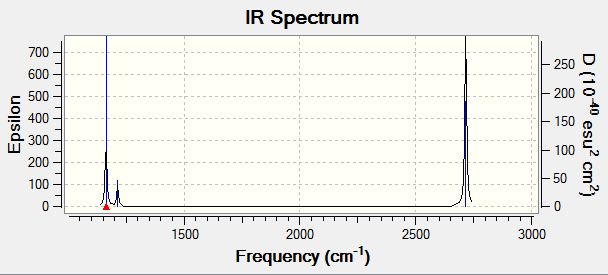
Vibrational spectrum for BH3
| Wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR Active? | type |
|---|---|---|---|---|
| 1163 | 93 | A2 | Yes | out-of-plane bend |
| 1213 | 14 | E' | Very slight | bend |
| 1213 | 14 | E' | Very slight | bend |
| 2582 | 0 | A1' | No | symmetric stretch |
| 2715 | 126 | E' | Yes | asymmetric stretch |
| 2715 | 126 | E' | Yes | asymmetric stretch |
There are only 3 peaks in the IR spectrum whereas there are 6 vibrational modes. This is because 1 is IR inactive as it is a symmetric stretch and there are two sets of degenerate overlapping vibrations.
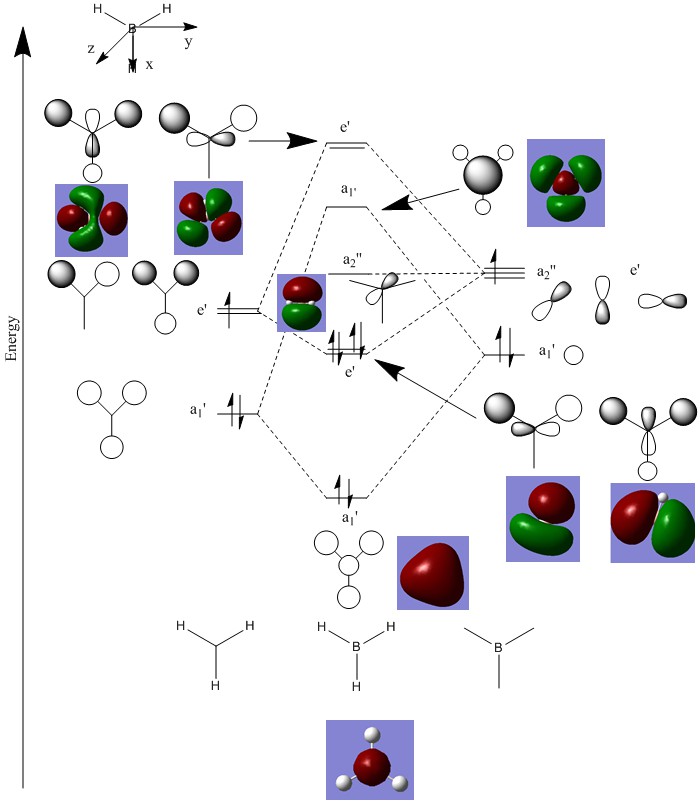
MO Diagram
The above diagram shows the LCAO predicted from a qualitative MO diagram and that predicted from Gaussview. The drawn MO diagram shows the position of the atomic orbitals within the predicted molecular orbitals whereas that predicted from Gaussview shows the overlap of the LCAO. In this MO diagram, there is no significant difference between the ordering of the MO's, however in more complex molecules qualitatively determining the ordering of certain orbitals can be difficult, especially when mixing is involved. Thus in most cases, a qualitative molecular orbital diagram is sufficient enough to understand bond orders, magnetic properties and reactivity.
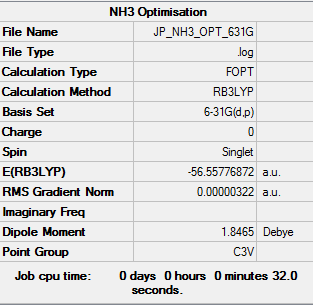
NH3
B3YLP/6-63G level
NH3Summary Table
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000016 0.001800 YES
RMS Displacement 0.000011 0.001200 YES
Frequency analysis log file File:JP NH3 FREQ.log
low frequencies
Low frequencies --- -8.5646 -8.5588 -0.0041 0.0455 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
NH3 |
Vibrational spectrum for NH3
| Wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR Active? | type |
|---|---|---|---|---|
| 1090 | 145 | A | Yes | out-of-plane bend |
| 1694 | 14 | E | Very slight | bend |
| 1694 | 14 | E | Very slight | bend |
| 3461 | 1 | A | Very slight | symmetric stretch |
| 3589 | 1 | E | Very slight | asymmetric stretch |
| 3589 | 1 | E | Very slight | asymmetric stretch |
There are only 3 peaks in the IR spectrum whereas there are 6 vibrational modes. This is because 1 is IR inactive as it is a symmetric stretch and there are two sets of degenerate overlapping vibrations.
NH3BH3 Adduct
B3YLP/6-63G level
Summary Table
Item Value Threshold Converged?
Maximum Force 0.000116 0.000450 YES
RMS Force 0.000060 0.000300 YES
Maximum Displacement 0.000574 0.001800 YES
RMS Displacement 0.000347 0.001200 YES
Low frequencies --- -0.0250 -0.0032 0.0004 17.1236 17.1259 37.1326 Low frequencies --- 265.7816 632.2034 639.3483
Frequency analysis log file File:JP NH3BH3 FREQ.log
Dissociation Energy
E(NH3)= -56.557769 au E(BH3)= -26.61532 au E(NH3BH3)= -83.22469 au
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] ΔE= -83.22469 -[-56.55777 - 26.61532] ΔE= -0.051560 au
ΔE= -135 kJ mol-1
The dative covalent bond strength is reasonable compared to other covalent bonds (C-C 345 kJ mol-1) but is still within an order of magnitude.
Ng611 (talk) 21:12, 15 May 2018 (BST) 'Reasonable' is too vague a comment. Is it strong, weak, etc? An order of magnitude is also too broad a definition, I would say that the bond value you cited is significantly higher than your one. Remember to also include a reference to the literature (paper or textbook ideally)
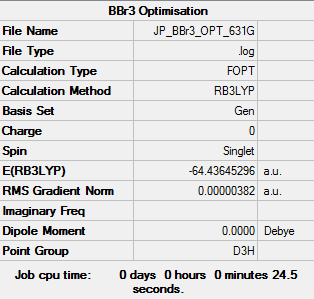
BBr3
B3YLP/GEN (PP LANL2DZ used for Br, 6-31G(d,p) for B)
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000023 0.001200 YES
Low frequencies --- -0.0116 0.0007 0.0040 56.4817 56.4817 57.0194 Low frequencies --- 145.1164 145.1199 200.4922
Frequency analysis log file: DOI:10042/202313
BBr3 |
Aromaticity
Benzene
6-31G B3YLP
Item Value Threshold Converged? Maximum Force 0.000194 0.000450 YES RMS Force 0.000084 0.000300 YES Maximum Displacement 0.000767 0.001800 YES RMS Displacement 0.000328 0.001200 YES
Low frequencies --- -16.9682 -14.6636 -14.6636 -0.0055 -0.0055 -0.0010 Low frequencies --- 414.1239 414.1239 620.9400
Frequency analysis log file File:JP Benzene FREQ.log
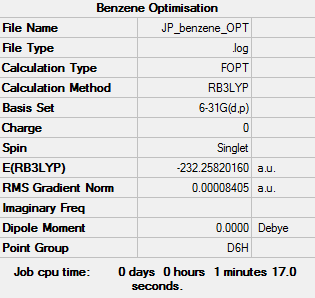
Benzene |
Borazine
6-31G B3YLP
Frequency analysis log file File:JP Borazine FREQ.log
Item Value Threshold Converged? Maximum Force 0.000194 0.000450 YES RMS Force 0.000061 0.000300 YES Maximum Displacement 0.000293 0.001800 YES RMS Displacement 0.000093 0.001200 YES
Low frequencies --- -3.0300 -0.0046 -0.0046 -0.0030 1.3740 1.3740 Low frequencies --- 289.7146 289.7146 404.4123
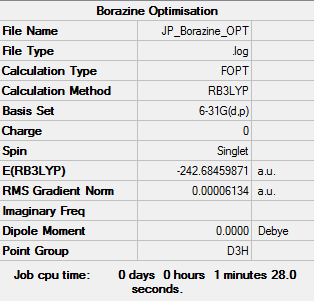
Borazine |
Charge Distribution Analysis
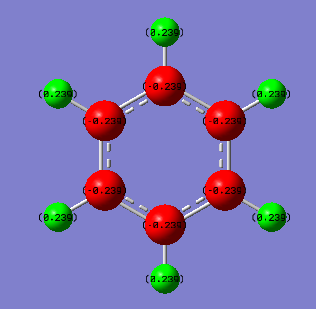
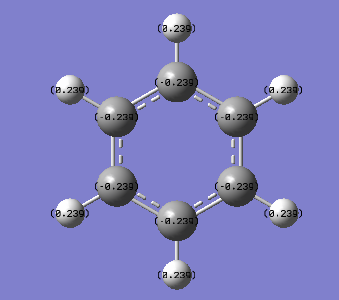
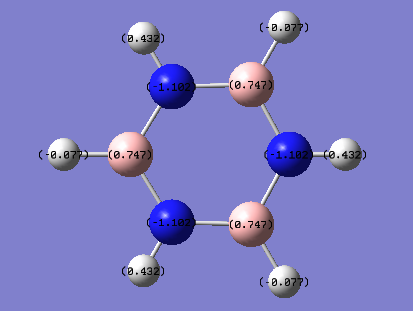
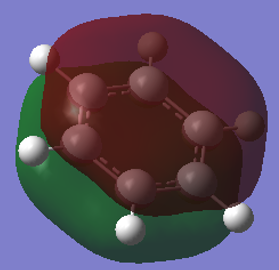
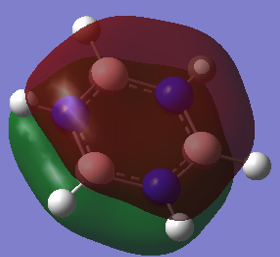
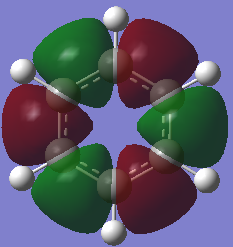
From the images above, it is seen that the charge is slightly negative and equally distributed among all the carbon atoms within benzene. In borazine, the charge is unevenly distributed with a larger negative charge being on the nitrogen atoms than that of carbon in benzene, and boron atoms having a positive charge. The larger charge difference in borazine is due to a greater difference in electronegativity than that in benzene. Since hydrogen is more electronegative than boron, the boron donates some electron density to the hydrogen hence its negative charge. The charges on the boron and the nitrogen agree with their reactivity whereby boron can act as a lewis acid and nitrogen as a lewis base.
Ng611 (talk) 21:15, 15 May 2018 (BST) Explaining that this equal distribution of charge is related to the symmetry of the molecule and that the sum of the partial charges is 0 would have improved this discussion further.
| Atom | Charge (arbitrary units) |
|---|---|
| C | -0.239 |
| H | -0.239 |
| Atom | Charge (arbitrary units) |
|---|---|
| B (B-H) | 0.747 |
| H | -0.077 |
| N | -1.102 |
| H (N-H) | 0.432 |
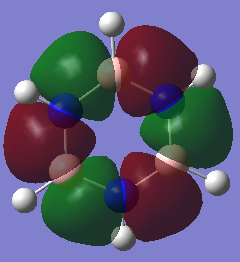
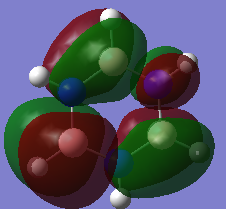
MO Comparison
Aromaticity traditionally arises from arises when there is a planar contiguous array of p orbitals orthogonal to the ring. leading to delocalisation above and below the plane of the ring. The equal bond lengths within borazine suggests aromaticity and delocalisation. The 6 pi electrons obey the 4n+2 rule thus agreeing with aromaticity. The lesser delocalisation within borazine compared to benzene reflects this reduced aromaticity. Computational methods have since proven that this model for aromaticity is not completely valid and can be associated with much more complex molecules such as polyacenes. The overlapping Pz description of aromaticity is sometimes not a good description due to the effect of the overlap of other orbitals such as those contributing to sigma bonds which may contribute to the delocalisation. The last factor is the charge/electronegativity differences leading to skewed molecular orbitals with unequal coefficients on each atom which affects the orbital overlaps and not just whether there is a Pz orbital orthogonal.
DOI:10.1002/chem.200700250
Ng611 (talk) 21:18, 15 May 2018 (BST) A well laid out/written report. Remember to check your work though: some job information recorded incorrectly (6-63G instead of 6-31G for BH3). Jmol missing for BH3NH3 adduct otherwise all of the calculation evidence was present. The section on aromaticity was very well done -- some more detail in your aromaticity discussion would have improved it further.