Jag115
NH3
Summary
| Property | Value |
|---|---|
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final energy E(RB3LYP) | -56.55776873au |
| RMS Gradient Norm | 0.00000485au |
| Point Group | C3V |
| Bond Length | 1.01798 Angstrom |
| H-N-H Bond Angle | 105.741° |
Item
| Item | Value | Threshold | Converged? | |
|---|---|---|---|---|
| Maximum | Force | 0.000004 | 0.00450 | Yes |
| RMS | Force | 0.000004 | 0.000300 | Yes |
| Maximum | Displacement | 0.00072 | 0.001800 | Yes |
| RMS | Displacement | 0.000035 | 0.001200 | Yes |
Predicted change in Energy=-5.986270D-10 Stationary point found.
Interactive Image
NH3 molecule |
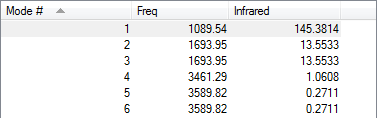
Molecule Vibrations
Display Vibrations Table for NH3
Questions
Applying the 3N-6, we would expect to see 6 modes which is what we see, as shown in the table above. Of the modes in the table above there are two degenerate pairs. Modes 2 and 3 are degenerate, both have a frequency of 1693.95, similarly modes 5 and 6 are also degenerate as both have a frequency of 3589.82. Modes 1, 2 and 3 are all bending vibrations and modes 4, 5 and 6 are all stretching vibrations. Both modes 1 and 4 are highly symmetric throughout the vibrations. Mode 1 is known as the umbrella mode, it can be called this as the nitrogen atom moves through the plane of the hydrogen atoms. There will be four different bands in gaseous ammonia.
On the nitrogen atom within the molecule the charge is found to be -1.125 and the charge on each of the hydrogen atoms 0.375. These values are expected as nitrogen is more electronegative than hydrogen so will withdraw electron charge density from within the covalent bonds towards itself, therefore nitrogen is delta negative and hydrogen is delta positive.
N2
Summary
| Property | Value |
|---|---|
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final energy E(RB3LYP) | -109.52412868au |
| RMS Gradient Norm | 0.00000000au |
| Point Group | D∞h |
| Bond Length | 1.09544 Angstrom |
Item
| Item | Value | Threshold | Converged? | |
|---|---|---|---|---|
| Maximum | Force | 0.000001 | 0.000450 | Yes |
| RMS | Force | 0.000001 | 0.000300 | Yes |
| Maximum | Displacement | 0.000000 | 0.001800 | Yes |
| RMS | Displacement | 0.000000 | 0.001200 | Yes |
Predicted change in Energy=-3.401019D-13 Stationary point found.
Interactive Image
N2 molecule, triple bond |
Molecule Vibrations
Display Vibrations Table for N2
As shown above there are no negative frequencies hence the molecule is fully optimised.
H2
Summary
| Property | Value |
|---|---|
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final energy E(RB3LYP) | -1.17853936au |
| RMS Gradient Norm | 0.00000017au |
| Point Group | D∞h |
| Bond Length | 0.74279 Angstrom |
Item
| Item | Value | Threshold | Converged? | |
|---|---|---|---|---|
| Maximum | Force | 0.000000 | 0.000450 | Yes |
| RMS | Force | 0.000000 | 0.000300 | Yes |
| Maximum | Displacement | 0.000000 | 0.001800 | Yes |
| RMS | Displacement | 0.000001 | 0.001200 | Yes |
Predicted change in Energy=-1.1640800D-13 Stationary point found.
Interactive Image
H2 molecule |
Molecule Vibrations
Display Vibrations Table for H2
None of the above values for frequency are negative therefore the molecule is fully optimised.
Harber Process
Bond Energies
| Species | Energy, amu |
|---|---|
| NH3 | -56.55776873 |
| 2*NH3 | -113.1155375 |
| N2 | -109.52412868 |
| H2 | -1.17853936 |
| 3*H2 | -3.53561808 |
Harber Process Calculation
From the above data and using Hess's law we can find the enthalpy for the Harber process, the below reaction to be:
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579074au
This value in au, equates to -146.48KJ/mol, hence the products are more stable than the reactants.
ClF
Summmary
| Property | Value |
|---|---|
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final energy E(RB3LYP) | -559.94269578au |
| RMS Gradient Norm | 0.00014211au |
| Point Group | C∞V |
| Bond Length | 1.99434 Angstrom |
Item
| Item | Value | Threshold | Converged? | |
|---|---|---|---|---|
| Maximum | Force | 0.000246 | 0.000450 | Yes |
| RMS | Force | 0.000246 | 0.000300 | Yes |
| Maximum | Displacement | 0.000433 | 0.001800 | Yes |
| RMS | Displacement | 0.000613 | 0.001200 | Yes |
Predicted change in Energy=-1.066054D-07 Stationary point found.
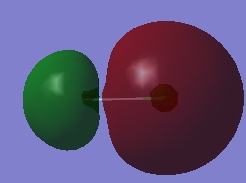
Relative Charges
The image above shows the relative charges of the molecule, the fluorine atom, on the left in light blue has a negative charge where as chlorine has a positive charge. Despite both atoms being electronegative, fluorine is more electronegative and hence withdraws electron charge density from the covalent bond and bears a negative charge.
Interactive Image
ClF molecule |
Molecule Vibrations
The vibrations shown above obey the 3N-5 rule for linear molecules, there are no negative energies for the molecule so we can conclude the molecule is fully optimised.
MOs of ClF
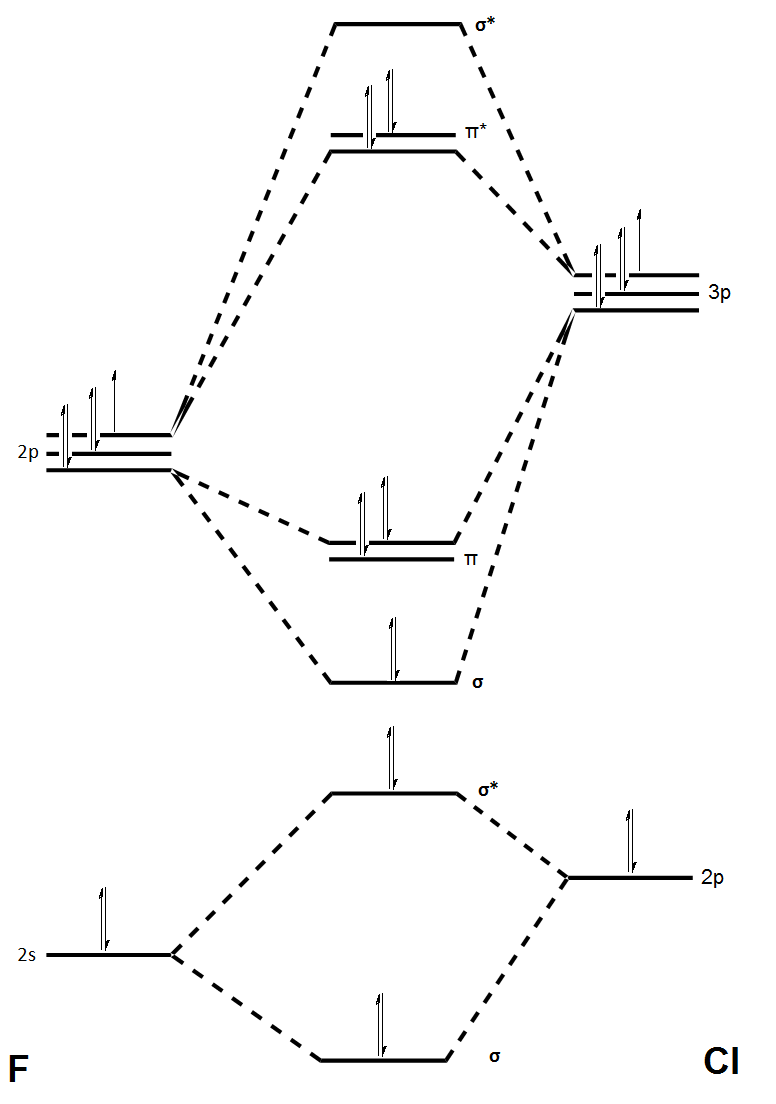
MO Diagram of ClF
The above diagram shows the MO overlaps for ClF from Cl and F and the relative energy levels of the bonding and anti-bonding overlaps of the AOs.