JJ1516MOY2
BH3
Calculation Overview
The following data were obtained using an RB3LYP calculation method with the 6-31G(d.p.) basis set; this followed an initial optimisation calculation using the same method with the 3-21G basis set.
Figure 1 presents the summary table for the data obtained from the frequency analysis performed on BH3. Figure 2 is an excerpt from the .LOG file confirming the convergence of the stated values (confirming a minimum energy structure of BH3/ successful optimisation). Figure 3 presents the calculated vibrations for BH3. Figure 4 displays the 3D interactive Jmol rendering for BH3.
Fig. 1 Summary of frequency calculation data
Item Value Threshold Converged? Maximum Force 0.000070 0.000450 YES RMS Force 0.000035 0.000300 YES Maximum Displacement 0.000274 0.001800 YES RMS Displacement 0.000137 0.001200 YES
Fig. 2 Confirmation of minimum energy structure
Low frequencies --- -26.6717 -24.6088 -24.6046 -0.0055 0.1852 0.4508 Low frequencies --- 1162.7586 1213.0226 1213.0253
Fig. 3 Excerpt of "low frequencies" from frequency.LOG file (cm-1)
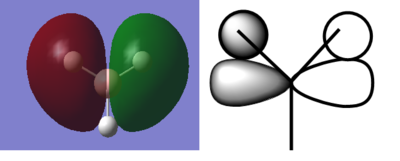
BH3 |
Fig. 4 3D interactive Jmol rendering for BH3
Link to BH3 frequency analysis log file
Molecular Vibrations
The table below presents a summary of the computed vibrational modes for the BH3 molecule. 3N - 6 = 6 (where N = 4) vibrational modes were found, as is expected for a non linear, tetratomic molecule.
| Vibration Number | Vibration Frequency / cm-1 | Absorption Intensity | IR Active | Vibration Type | Symmetry |
|---|---|---|---|---|---|
| 1 | 1162 | 93 | ACTIVE | Angle Deformation | Symmetric |
| 2 | 1213 | 14 | ACTIVE | Angle Deformation | Asymmetric |
| 3 | 1213 | 14 | ACTIVE | Angle Deformation | Asymmetric |
| 4 | 2583 | 0 | INACTIVE | Bond Stretch | Symmetric |
| 5 | 2716 | 126 | ACTIVE | Bond Stretch | Asymmetric |
| 6 | 2716 | 126 | ACTIVE | Bond Stretch | Asymmetric |
Table 1 The molecular vibrations of BH3
It has been found that the vibrations numbered 2 & 3 (1213 cm-1) form a degenerate pair of vibrations as do vibrations 5 & 6 (2716 cm-1). These correspond to the absorption of photons of 0.15 eV and 0.34 eV respectively. The calculated intensity of vibration 4 is zero; thus, the vibrational mode is deemed IR inactive. Upon inspection of the molecule animation for this vibration, it was indeed found to be a symmetric bond stretch which resulted in no change in the molecule's dipole moment. Furthermore, the computed IR spectrum (Figure 5, below) shows no absorption peak at 2583 cm-1. Three absorption peaks are visible on the spectrum, corresponding to the three sets of vibration frequencies (since there are two degenerate pairs within the five IR active vibrations).
Fig. 5 Computed IR spectrum
Molecular Orbitals

A qualitative molecular orbital (MO) diagram for BH3 is presented (Figure 6, right). The MOs of BH3 have been composed from the valence orbitals of boron and a fictitious H3 symmetry fragment (composed of 3 H atoms). MO theory predicts that some MOs will contribute more significantly to the overall bonding in a molecule; this serves to explain the omission of the occupied yet non-valence orbital of B (the a1', 1s orbital) as its very low energy relative to the fragment MOs shown prevents its involvement in any significant bonding interaction. Given that there are no MOs of the same symmetry belonging to different, bonding-antibonding pairs, no MO mixing is expected to occur / is included in the diagram.
The frequency calculation performed with Gaussian under the specified method and basis set has also provided theoretical MOs for BH3. The comparison of the computed MOs and the qualitative MO diagram enables the determination of the accuracy (and utility) of drawing MO diagrams. Figure 7 presents the computed MOs and their energy in energy atomic units (Hartrees, Eh, 1Eh = 4.3567482(26) x 10-18J).
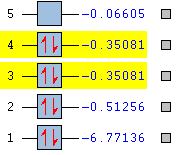
Fig. 7 Theoretical energy and occupancy of the first 5 MOs of BH3
Figure 7 also shows that the electron occupancy of the energy levels in the computational model agrees with that of the qualitative treatment (filled core MO, all bonding orbitals fully filled, no occupation of non-/anti-bonding MOs).
The first MO (bottom) is significantly lower in energy than the rest (-6.77 Eh). This MO is spherically symmetric, has no nodes, overlaps with no other orbitals and is centered on the B atom. Therefore, it is sensible to suggest that this is the B 1s atomic orbital which, as mentioned, is not involved with bonding. The third and fourth computed MOs (highlighted) form a degenerate pair; this agrees with the qualitative treatment of the bonding in BH3 as the third and fourth MOs (e1') on the diagram are found to be degenerate. Figures 8 and 9 juxtapose the theoretical degenerate MOs against the qualitative e1' orbitals as predicted by MO theory.
The above graphics show that the expected regions of increased electron density due to in-phase overlap are indeed predicted by the theoretical calculation. The shape of the theoretical orbitals is well reproduced by the qualitative treatment.
Typically, the HOMO and LUMO govern the reactivity of a molecule; often, particular interest is afforded to these MOs. Below (Figure 10) the theoretical LUMO of BH3 is presented alongside the qualitative prediction.
From the above comparisons, it is clear to see that the MOs produced from the overlap of fragment/atomic orbitals as predicted qualitatively by MO theory are wholly supported by the results of the computational MO calculation. In the case of the unoccupied antibonding orbitals, the computed orbital shapes are distorted with respect to the qualitative predictions as the former accounts for the repulsion between electrons in out-of-phase orbitals while the latter does not. Overall, the computational results have validated the effectiveness of the MO diagram (for small, highly symmetric molecules such as BH3).
H3NBH3
Bonding
Ammonia-borane results from the association of ammonia with borane, an acid base pair (BH3 and NH3 respectively). The bonding in ammonia-borane (H3NBH3) may be rationalised as the donation of a lone electron pair from the N atom in ammonia to the electron-deficient B centre in borane. In other words, a dative covalent bond is formed between N and B. However, it must be said that the idea of dative bonding is merely an aid to equating the total electron count on both sides of the following equation (formation of ammonia-borane): .
The electron distribution about a dative covalent bond is no different and is totally indistinguishable from a conventionally termed covalent bond.
With this model, the computation of the reaction energy is a facile process: the reaction energy will be given by the difference in energy between the products and reactants or the energy of the N-B bond formed. Below, the conditions and results of the frequency analyses of (optimised) NH3 and H3NBH3 are presented. This data has been used together with the data acquired for BH3 to determine thea reaction energy.
NH3
The following data were obtained using an RB3LYP calculation method with the 6-31G(d.p.) basis set. Figure 11 presents the summary for the frequency calculation for optimised NH3. Figure 12 is an excerpt from the .LOG file confirming the convergence of the frequency calculation. Figure 13 presents the calculated vibrations of NH3.
Fig. 11 Summary of frequency calculation data
NB: A C3v point group was obtained before the frequency calculation was performed. A link to the summary of the C3v molecule may be found here
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000013 0.001800 YES RMS Displacement 0.000007 0.001200 YES
Fig. 12 Confirmation of minimum energy structure
Low frequencies --- -0.0138 -0.0032 0.0018 7.0783 8.0932 8.0937 Low frequencies --- 1089.3840 1693.9368 1693.9368
Fig. 13 NH3 vibrations obtained
NH3 |
Fig. 14 3D interactive Jmol rendering for NH3.
Link to NH3 frequency analysis log file
H3NBH3
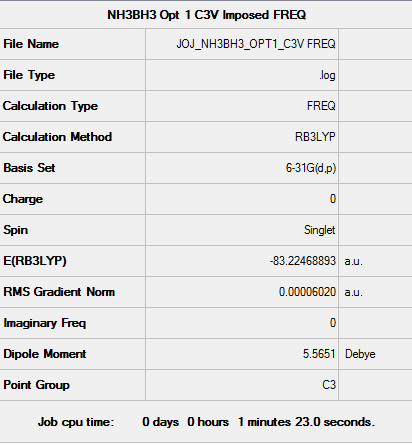
The following data were also obtained using an RB3LYP calculation method with the 6-31G(d.p.) basis set. Figure 15 presents the summary for the frequency calculation performed on the optimised adduct. Figure 16 is an excerpt from the .LOG file confirming the convergence of the frequency calculation. Figure 17 presents the calculated vibrations of H3NBH3.
Fig. 15 Summary of frequency calculation data
NB: A C3v point group was obtained before the frequency calculation was performed. A link to the summary of the C3v molecule may be found here
Item Value Threshold Converged? Maximum Force 0.000115 0.000450 YES RMS Force 0.000060 0.000300 YES Maximum Displacement 0.000569 0.001800 YES RMS Displacement 0.000345 0.001200 YES
Fig. 16 Confirmation of minimum energy structure
Low frequencies --- -0.0250 -0.0031 0.0007 17.1475 17.1498 37.2156 Low frequencies --- 265.8048 632.2133 639.3570
Fig. 17 H3NBH3 vibrations obtained
H3NBH3 |
Fig. 18 Jmol rendering for H3NBH3
Link to H3NBH3 frequency analysis log file
Reaction Energy
As previously stated, the reaction energy (ΔE) is taken to be the energy of the N-B bond present in the product of the association of ammonia to borane. Thus ΔE is calculable by E(NH3BH3)-[E(NH3)+E(BH3)]. The values of the terms in this equation are presented below. These values have been rounded to accommodate the approximate 10 kJ·mol-1 uncertainty in the computational calculations.
- E(BH3)
- -26.615 Eh = -69880 kJ·mol-1
- E(NH3)
- -56.558 Eh = -148490 kJ·mol-1
- E(NH3BH3)
- -83.224 Eh = -218510 kJ·mol-1
- ΔE = -140 kJ·mol-1
Comparing this value to the experimentally determined bond energy of an isoelectronic structure of similar size (i.e. ethane) suggests the N - B is somewhat weak. Spectroscopic methods have been employed to determine the C - C bond energy in ethane as -377.4 kJ·mol-1[1]. This is almost three times as great as the calculated ΔE.
BBr3
Psuedo-potentials and Mixed Basis Sets
Given the presence of the heavy Br atoms in boron tribromide, a revision to the prior calculation method is needed. In Br, a large number of electrons occupy several atomic orbitals from n =1 to n = 4 (where n is the principal quantum number). Atomic orbitals of low n values penetrate more deeply into the "core" region of the atom and experience a strong electrostatic interaction due to the atom's nuclear charge. Owing to their low energy, these electrons are mostly uninvolved in chemical bonding. However, these electrons do screen the electrons residing in atomic orbitals of high n, causing them to experience a decreased, effective nuclear charge. Additionally, there are significant relativistic effects affecting the Br electrons which arise as the core electrons approach relativistic speeds (due to the high atomic number/nuclear charge). To account for these effects, a pseudo-potential or effective core potential is applied to the Br atoms. The 6-31G basis set is applied to the lighter B atom in which the aforementioned effects are less or even insignificant. Applying the more computationally complex method and basis set to the Br atom while retaining the 6-31G basis set for B enables an increase in accuracy with less of an increase in computational expense. This application of a mixed basis set promotes manageable and practical calculation times.
Smf115 (talk) 12:47, 25 May 2018 (BST)Excellent information about the pseudopotential and clear awareness of the method used for each calculation and why throughout.
Calculation Overview
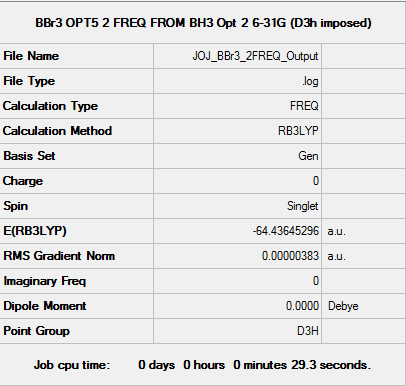
The following data were obtained using an RB3LYP calculation method. The 6-31G basis set has been employed for the B atom and the LANL2DZ basis set and pseudo-potential used for the Br atoms.
Figure 19 presents the summary for the frequency calculation performed on the optimised BBr3 molecule. Figure 20 is an excerpt from the .LOG file confirming the convergence of the frequency calculation. Figure 21 presents the calculated vibrations of BBr3 while Figure 22 presents a 3D interactive rendering of the molecule.
Fig. 19 Summary of the frequency calculation
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000018 0.001200 YES
Fig. 20 Confirmation of minimum energy structure
Low frequencies --- -0.0137 -0.0064 -0.0046 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
Fig. 21 Vibrations obtained for BBr3
BBr3 |
Fig. 22 3D interacting Jmol rendering of BBr3
Link to BBr3 frequency analysis log file
DSpace Link http://hdl.handle.net/10042/202434
Smf115 (talk) 12:51, 25 May 2018 (BST)Great first section overall with a lot of additional information and a great introduction to the ammonia-borane subsection. To improve just be aware that energy values are always reported to the nearest kJ/mol, even though they are not necessarily accurate to this level, and the symmetry assignments were missing from the IR table.
Project: Aromaticity
Calculation Overview
Below, the results of the frequency calculations performed on benzene and borazine are featured. Both calculations were carried out with an RB3LYP calculation method with the 6-31G(d.p.) basis set.
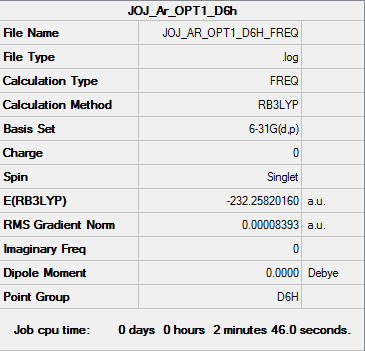
Fig. 23 Summary of the frequency calculation
Item Value Threshold Converged? Maximum Force 0.000194 0.000450 YES RMS Force 0.000084 0.000300 YES Maximum Displacement 0.000767 0.001800 YES RMS Displacement 0.000328 0.001200 YES
Fig. 24 Confirmation of minimum energy structure
Low frequencies --- -16.9682 -14.6636 -14.6636 -0.0055 -0.0055 0.0001 Low frequencies --- 414.1239 414.1239 620.9400
Fig 25 The vibrations obtained for benzene
Benzene |
Link to benzene frequency analysis log file
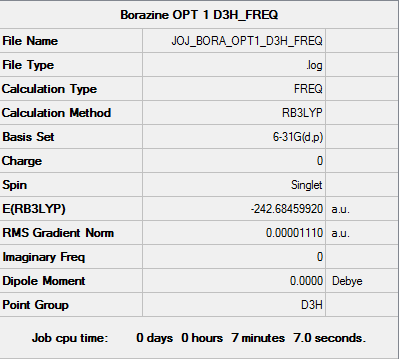
Fig. 26 Summary of the frequency calculation
Item Value Threshold Converged? Maximum Force 0.000029 0.000450 YES RMS Force 0.000011 0.000300 YES Maximum Displacement 0.000084 0.001800 YES RMS Displacement 0.000036 0.001200 YES
Fig. 27 Confirmation of minimum energy structure
Low frequencies --- -4.8064 -4.7828 -4.0590 -0.0030 0.0035 0.0050 Low frequencies --- 289.6877 289.6884 404.4509
Fig 28 The vibrations obtained for borazine
Borazine |
Link to borazine frequency analysis log file
Charge Distribution
Both borazine and benzene are neutral molecules. Thus, it is expected that the sum of all the partial charges is equal to zero.
The partial charges on the atoms arise from the polarisation of the covalent bonds due to the differences in electronegativity of the atoms of said bonds. The greater the difference in electronegativity between the two atoms in a bond, the greater the polarisation / ionic character of the bond. This results it greater partial charges residing on each atom.
In benzene, C - C and C - H bonds are present. According to the Pauling electronegativity scale, C has an electronegativity (χ[2]) of 2.54 while χH = 2.3. The C - H bonds are only slightly polar due to the similarity in these values; small negative and small positive charges reside on the C and H atoms respectively. The C - C bonds are not polarised.
In borazine, B - N, N - H and B- H bonds are present. The values of χ are as follows:
- χB = 2.05
- χH = 2.30
- χN = 3.07
From the above values, a number of predictions may be made. The difference in electronegativity (Δχ) between C and H is less than that between B and H and N and H. Thus, it is predicted that the N - H and B - H bonds will be more polarised than the C - H bonds. Additionally, while the ring atoms in benzene are all identical, the B - N bonds in borazine are highly polarised: as each B atom is bonded to two N atoms of much greater electronegativity (and vice versa), a high positive/negative partial charge is expected to reside on the B/N atoms.
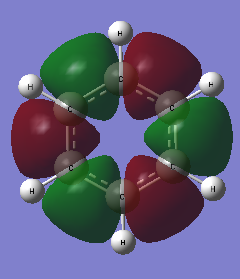
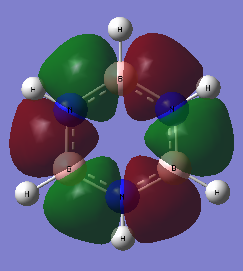
Figure 29 displays the NBO charge distributions for both molecules with a charge scale for comparison. The numerical values of the partial charges are listed in Table 2
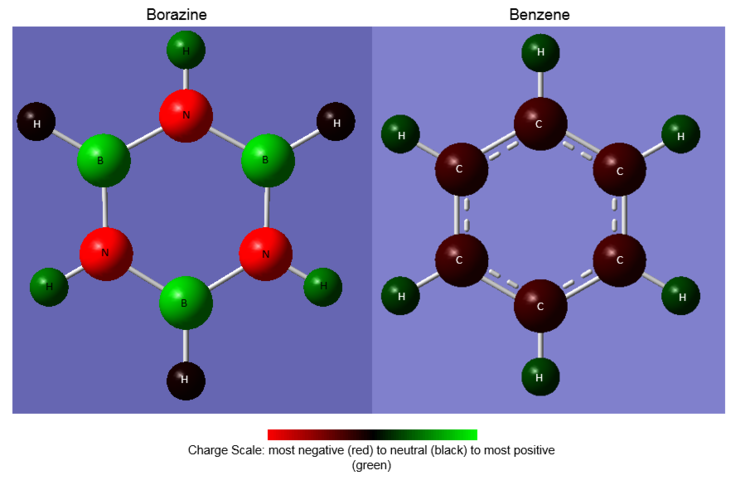
Fig 29 The charge distributions of benzene and borazine
| Molecule | Atom | Frequency | Bond(s) | Partial Charge / e |
|---|---|---|---|---|
| Benzene | C | 6 | C - C / C - H | -0.239 |
| H | 6 | C - H | 0.239 | |
| Borazine | N | 3 | B - N - B / N - H | -1.102 |
| B | 3 | N - B - N / B - H | 0.747 | |
| H | 3 | B - H | -0.077 | |
| H | 3 | N - H | 0.432 |
For both molecules, the sum of partial charges is equal to zero. Indeed it has been found that the bonds in borazine are more polarised than those in benzene; greater partial charges (measured in units of e, atomic unit of charge / magnitude of electron charge / elementary charge) reside on the ring atoms in borazine than on the ring atoms in benzene. In other words, there is a much greater delocalisation of charge around the ring atoms in benzene than in borazine.
MO Analysis
Table 3 presents a series of comparisons between analogous molecular orbitals of benzene and borazine as obtained from the frequency calculation.
Table 3 Comparison of benzene and borazine MOs
Smf115 (talk) 17:41, 26 May 2018 (BST)Good range of orbitals selected and the character and type of the MOs has been clearly identified. To improve, consider a few more details which could be mentioned such as the symmetries of the MOs or the AOs which contribute.
Despite MOs #14 & #15 being composed of p orbitals which are not parallel to one another / orthogonal to the plane of their respective rings, they provide a pathway for electron delocalisation around the rings. This suggests that there is some need to expand the traditional, Hückel criteria for aromaticity.
Smf115 (talk) 17:41, 26 May 2018 (BST)Overall, a very good start to the project section with a great charge analysis, including clearly presented electronegativities, NBO charges and diagrams. However, the aromaticity question hasn't really been considered. Well presented report with a few mistakes throughout.
Aromaticity
The investigation into the MOs and charge distribution of borazine and benzene, two isoelectronic molecules with distinct reactivities has yielded a surprising amount of similarity. While many of the MOs bear incredible resemblances, the polarity of the bonds within the borazine ring and the even charge distribution on benzene (visible on many MOs) enables the deduction that aromaticity may be defined by a broader range of criteria than Huckel's rules (which depend upon the contiguous p orbital array).
References
1) Luo, Y., 2002. Handbook of Bond Dissociation Energies in Organic Compounds. CRC Press
2) Pauling, L., The Nature of the Chemical Bond, Third Edition, Cornell University Press, Ithaca, New York, 1960