It:sitagliptin page
Sitagliptin ( Januvia tm )
Brief Definition :
Sitagliptin is a relatively new drug (Approved by FDA 17/10/2006) used for treating type 2 Diabetes Diabetes Mellitus.
| Sitagliptin | |
|---|---|
| |
| IUPAC Systematic name | |
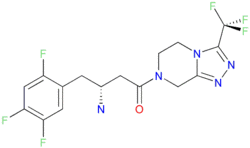
| (3R)-3-amino-1-[9-(trifluoromethyl)-1,4,7,8-tetrazabicyclo[4.3.0]nona-6,8-d ien-4-yl]-4-(2,4,5-trifluorophenyl butan-1-one | |
| Other name | |
| Januvia | |
| Indentifiers | |
| ATC Code | - |
| CAS number | - |
| PubChem (CID) | 4369359 |
| SMILES | FC1=CC(F)=C(F)C=C1C[C@@H]([N])CC(N2CC3=NN=C([C@@](F)(F)F)N3CC2)=O FC1=CC(F)=C(F)C=C1C[C@@H]([N])CC(N2CC3=NN=C([C@@](F)(F)F)N3CC2)=O |
| Chemical Data | |
| Molecular formula | C16H15N5F6O |
| Molar mass | 407.314 g/mol |
| Pharmacokinetic Data | |
| Bioavailability | 87% |
| Protein Binding | 38% |
| Metabolism | Hepatic (CYP3A4- and CYP2C8-mediated) |
| Half life | 8 to 14 hours |
| Excretion | Renal (80%) |
| Therapeutic considerations | |
| Pregnancy cat. | B (US) |
| Legal status | Rx-only |
| Routes | Oral |
What is Sitagliptin?
The new drug to fight type 2 diabetes Sitagliptin has shown from the trials that it reduces glucose in blood, improves beta-cell function and can help patients control their weight. This drug was only approved for use on type 2 diabetes on 17th October 2006. The sitagliptin worked best to help patients with type 2 diabetes when added to metformin or TZDs. The drug helps to control inculin release due to beta-cell disfunction, and controls the production of glucose by the liver which is uncontrollable when there is alpha and beta cell disfunction. [1]
How Sitagliptin works
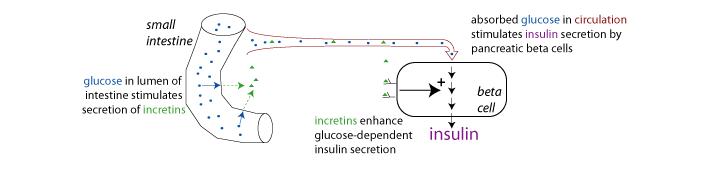
Sitagliptin is a DPP-4 inhibitor which is believed to slow the inactivation of incretin (a type of gastrointestinal hormones) hormones and the concentration of these particular hormones are actually increased by Januvia. Incretin hormones (see diagram 1), including glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), are released by the intestine throughout the day, and levels are increased in response to a meal. The source of the diabetic problem is that the enzyme DPP-4 inactivates these hormones which are necessary for the handling of glucose in the blood. When blood glucose concentrations are normal or elevated, GLP-1 and GIP increase insulin synthesis and release from pancreatic beta cells by intracellular signaling pathways involving cyclic AMP. GLP-1 also lowers glucagon secretion from pancreatic alpha cells, leading to reduced hepatic glucose production. JANUVIA increases insulin release and decreases glucagon levels in the circulation in a glucose-dependent manner. Sitagliptin demonstrates selectivity for DPP-4 and does not inhibit DPP-8 or DPP-9 activity in vitro at concentrations approximating those from therapeutic doses.
Side effects
Although only a new drug the side effects that have been found when in phase 2 and 3 of testing are
- stuffy or runny nose and sore throat
- upper respiratory infections
- headaches [1]
Structure of Sitgliptin
3D Structures of Sitagliptin
In order to see the 3D structure in anaglyph 3D mode, special Red/Blue anaglyph glassess will be needed.
| Jmol Structure | ||
|---|---|---|
|
|
Pharmaceutical Information
Dosage and Administration

- The recommended dose of JANUVIA is 100 mg once daily as monotherapy or as combination therapy with metformin or a PPARg agonist (e.g., thiazolidinediones). JANUVIA can be taken with or without food.
Patients with Renal Insufficiency
- For patients with mild renal insufficiency (creatinine clearance [CrCl] ³50 mL/min, approximately corresponding to serum creatinine levels of £1.7 mg/dL in men and £1.5 mg/dL in women), no dosage adjustment for JANUVIA is required.
- For patients with moderate renal insufficiency (CrCl ³30 to <50 mL/min, approximately corresponding to serum creatinine levels of >1.7 to £3.0 mg/dL in men and >1.5 to £2.5 mg/dL in women), the dose of JANUVIA is 50 mg once daily.
- For patients with severe renal insufficiency (CrCl <30 mL/min, approximately corresponding to serum creatinine levels of >3.0 mg/dL in men and >2.5 mg/dL in women) or with end-stage renal disease (ESRD) requiring hemodialysis or peritoneal dialysis, the dose of JANUVIA is 25 mg once daily. JANUVIA may be administered without regard to the timing of hemodialysis.
- Because there is a need for dosage adjustment based upon renal function, assessment of renal function is recommended prior to initiation of JANUVIA and periodically thereafter. Creatinine clearance can be estimated from serum creatinine using the Cockcroft-Gault formula. [See CLINICAL PHARMACOLOGY .]
Dosage Forms and Strengths
- 100 mg tablets are beige, round, film-coated tablets with "277" on one side.
- 50 mg tablets are light beige, round, film-coated tablets with "112" on one side.
- 25 mg tablets are pink, round, film-coated tablets with "221" on one side.
Pharmakinetics
- The pharmacokinetics of sitagliptin has been extensively characterized in healthy subjects and patients with type 2 diabetes. After oral administration of a 100 mg dose to healthy subjects, sitagliptin was rapidly absorbed, with peak plasma concentrations (median Tmax) occurring 1 to 4 hours postdose.
Plasma AUC of sitagliptin increased in a dose-proportional manner. Following a single oral 100 mg dose to healthy volunteers, mean plasma AUC of sitagliptin was 8.52 mM·hr, Cmax was 950 nM, and apparent terminal half-life (t1/2) was 12.4 hours. Plasma AUC of sitagliptin increased approximately 14% following 100 mg doses at steady-state compared to the first dose. The intra-subject and inter-subject coefficients of variation for sitagliptin AUC were small (5.8% and 15.1%). The pharmacokinetics of sitagliptin was generally similar in healthy subjects and in patients with type 2 diabetes.
Absorption
- The absolute bioavailability of sitagliptin is approximately 87%. Because coadministration of a high-fat meal with JANUVIA had no effect on the pharmacokinetics, JANUVIA may be administered with or without food
Metabolism
- Approximately 79% of sitagliptin is excreted unchanged in the urine with metabolism being a minor pathway of elimination.
Following a [14C]sitagliptin oral dose, approximately 16% of the radioactivity was excreted as metabolites of sitagliptin. Six metabolites were detected at trace levels and are not expected to contribute to the plasma DPP-4 inhibitory activity of sitagliptin. In vitro studies indicated that the primary enzyme responsible for the limited metabolism of sitagliptin was CYP3A4, with contribution from CYP2C8.
Excretion
- Following administration of an oral [14C]sitagliptin dose to healthy subjects, approximately 100% of the administered radioactivity was eliminated in feces (13%) or urine (87%) within one week of dosing. The apparent terminal t1/2 following a 100 mg oral dose of sitagliptin was approximately 12.4 hours and renal clearance was approximately 350 mL/min.
- Elimination of sitagliptin occurs primarily via renal excretion and involves active tubular secretion. Sitagliptin is a substrate for human organic anion transporter-3 (hOAT-3), which may be involved in the renal elimination of sitagliptin. The clinical relevance of hOAT-3 in sitagliptin transport has not been established. Sitagliptin is also a substrate of p-glycoprotein, which may also be involved in mediating the renal elimination of sitagliptin. However, cyclosporine, a p-glycoprotein inhibitor, did not reduce the renal clearance of sitagliptin.
Type 2 Diabetes
Type 2 diabetes is the most common form of diabetes. In type 2 diabetes, either the body does not produce enough insulin or the cells ignore the insulin. Insulin is necessary for the body to be able to use sugar. Sugar is the basic fuel for the cells in the body, and insulin takes the sugar from the blood into the cells. When glucose builds up in the blood instead of going into cells, it can cause two problems:
- Right away, your cells may be starved for energy.
- Over time, high blood glucose levels may hurt your eyes, kidneys, nerves or heart.
Associated Conditions
- Conditions associated with type 2 diabetes include hyperglycemia and hypoglycemia