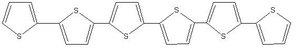
It:sexithiophene
Appearance
Sexithiophene - The 'Sexi' Molecule
Brief Description
- The Sexithiophene molecule consists of 6 thiophene subunits which contains conjugated double bonds. within This makes the molecule an electrical conductor and renders it useful for application in Organic Polymer Electronics.
Electronic Properties
- The thiophene oligomers (nT, n standing for number of thiophenes) were first synthesised around 60 years ago and initial investigations into these oligomers showed that the 4T structure was useful as a insecticide. More recently the applications of these species have moved towards use in electronic devices such as Field –Effect Transistors (FET) and Light Emitting Diodes (LED). The Sexithiophene 6T oligomers derivatives have reported the highest Field-Effect mobility for organic-based FET devices known to date.
Applications of sexithiophene

- Sexithiophene is a conjugated molecule which can be used as an organic semiconductor. In its solid state sexithiophene is a highly ordered material with a high degree of crystallinity even in a thin film explaining the compounds excellent transport properties.Organic semi conductors play a vital role in the development of new applications in the field known as “plastic” electronics. They have a large number of advantages in comparison to silicon and other mineral semiconductors (which are normally used to make up transistors). Organic semiconductors (unlike mineral semiconductors) can be deposited in a thin film on to a surface using relatively inexpensive techniques; they can also be applied to flexible support systems. The potential uses of the organic semiconductor are vast including areas such as integrated circuits, display devices (flat screens) and logic microelectronics.
Synthesis of sexithiophene
Sexithiophene is a polythiophene (a polymer of thiophene which is an aromatic sulphur heterocycle). Polythiophenes are synthesised either:
- electrochemically by applying a potential across a solution of the monomer to be polymerised.
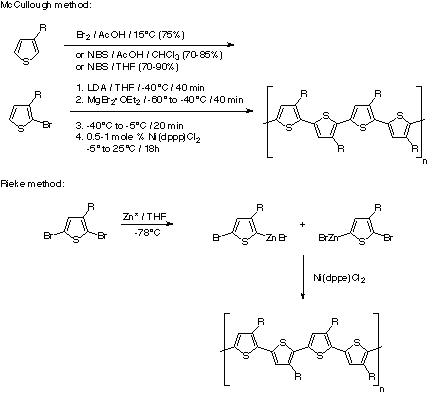
- chemically by using oxidants or cross-coupling catalysts.Two methods of chemical synthesis are the McCullough and Rieke methods. The McCullough method involves a selective bromination which produces a 2-bromo-3-alkylthiophene. This compound then undergoes a transmetallation and kumada cross-coupling using a nickel catalyst to form the polythiophene. The Rieke method is where 2, 5-dibromo-3-alkylthiophene is reacted with “Rieke zinc" to produce a mixture of organometallic isomers which is then treated with Ni(dppe)Cl2 to form the polythiophene.
(reactions schemes taken from http://en.wikipedia.org/wiki/Polythiophene)