It:limonene
| Limonene | |
|---|---|
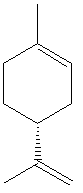
| Chemical name | 1-methyl-4-prop-1-en-2-yl-cyclohexene |
| Synonyms | 4-isopropenyl-1-methylcyclohexene Racemic: DL-limonene; dipentene |
| Chemical formula | C10H16 |
| Molecular mass | 136.24 g/mol |
| Melting point | -95.2 °C |
| Boiling point | 176 °C |
| Density | 0.8411 g/cm3 |
| Refractive index | 1.4730 |
| Flash point | 50°C |
| SMILES | CC1=CCC(C(=C)(C))CC1 |
| MSDS | |
Limonene
Limonene is an organic molecule, one enantiomer of which smells extremely like oranges (D-limonene, the (R)- enantiomer), being the active ingredient in citrus. The other enantiomer smells like something of a cross between pine and turpentine, and unsurprisingly the molecule is classed as a "turpene".
Uses
Limonene has long been used to give an orange or a lemon flavour to food products (depending on what it's used with), and also to give that lovely "lemon fresh" smell to things like fairy liquid and bleach. Recently however, it has been realised that it can be used as a solvent for cleaning products on its own, leading to an explosion in "more natural" cleaning products - Mr Muscle Orange Action is a good example.(Despite the excellent marketing point that being a "more natural" product is, the reason limonene is now being used as solvent is that it is much less hazardous to health than some things that have been used as solvents in cleaning products in the past...)
Industrial Manufacture
Industrially, limonene is extracted as an oil from the rinds of citrus fruits (most commonly oranges, lemons and limes) and as such is produced as a by-product of the juice industry. Two grades of limonene oil are obtained; the first, food grade oil, is extracted by pressing the fruit rinds. This oil is then purified by distillation (some of the distillates can also be recovered for sale, of use as flavourings or fragrances). After this pressing process, the peel is then steamed heated; when the steam is condensed, a layer of limonene oil is left floating on its surface. This is known as technical grade limonene, and is what is used in cleaning products.
Sythesis
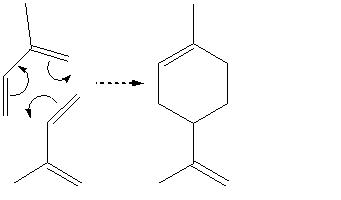
The simplest way to synthesize limonene, and the one used industrially, is by the Diels-Alder reaction (in this case a dimerization as well) between two molecules of isoprene (this leads to its synonym, dipentene):
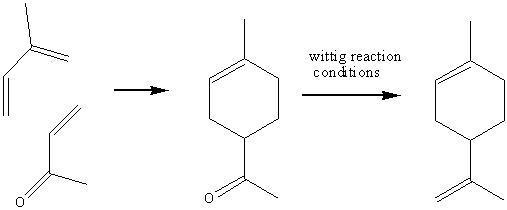
However, the problem with this synthetic route is that it produces both enantiomers, leading to problems of separation and isolation. Fortunately it has been discovered that reacting isoprene with mthyl vinyl ketone, followed by a wittig reaction to convert the carbonyl group to a c=c double bond:
This reaction can be sped up and made more regio-slective by the addition of a lewis acidic catalyst, such as AlCl3. The product mixture is still racemic, but much less so than with the dimerization reaction; roughly 75% of the useful enantiomer instead of just under 50%.