It:Ziegler-Natta
Introduction
A Ziegler – Natta catalyst is a method of producing unbranched, stereoregular vinyl polymers, such as linerar unbranched polyethylene and isotactic polypropylene. Ziegler – Natta catalysts are typically based on titanium chlorides and organometallic trialkyl aluminium based co-catalyst.
The catalyst was discovered by a German Chemist Karl Ziegler (1898-1973) in 1953. He produced a tougher polymer with higher melting point than polyethylene which was produced at that time using a resin catalyst. The catalyst was modified by an Italian chemist Giulio Natta (1903-1979) in 1953, a new type of plastic was produced which he called ‘isotactic’ polymers. Karl Ziegler and Giulio Natta were awarded the Nobel Prize in Chemistry in 1963 for the discovery and its uses to produce stereoregular polymers.
The type of Ziegler-Natta catalysts used in the synthesis could be:
- Homogeneous - the aluminium cocatalyst is not required in the polymerization
- Heterogeneous - the cocatalyst is required in the polymerisation as shown in the isotactic polymerization
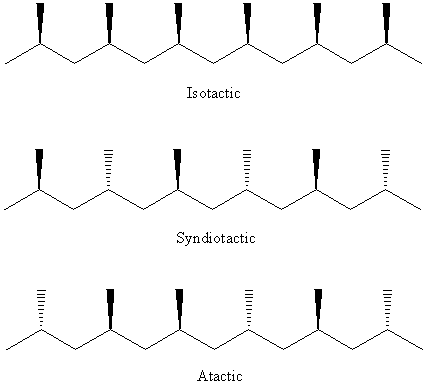
Different types of Ziegler-Natta catalysts used leads to stereospecific polymerization of alkenes, e.g. isotactic polypropylene (or polypropene) from titanium-based catalyst, syndiotatic polypropylene from vanadium-based catalyst4 .
The structure of syndiotactic, isotactic and atactic polypropylene:
Preparation of Catalyst
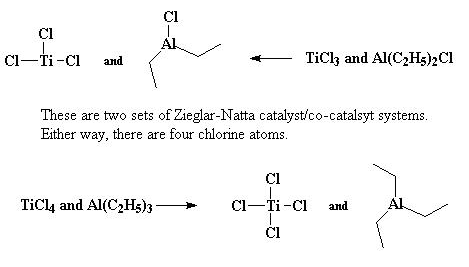
Most of the time the catalyst and co-catalysts pair are TiCl3 and Al(C2H5)2Cl, or TiCl4 with Al(C2H5)3.
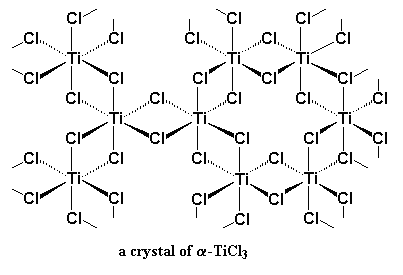
In this case, the TiCl3 and Al(C2H5)2Cl system will be discussed. TiCl3 can arrange itself into a number of crystal structures. The one that is interested in is called α- TiCl3.
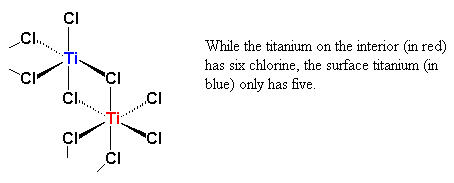
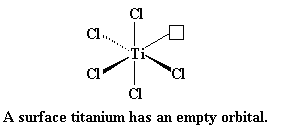
As it can be seen, there are six chlorine atoms coordinated to each titanium atom, with an octahedral geometry. In the interior of the crystal, each titanium is surrounded by six chlorines, but on the surface, a titanium atom is surrounded on one side by five chlorine atoms, and the other side by empty space.
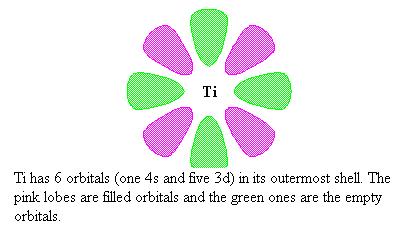
Titanium has six empty orbitals, one 4s and five 3-d orbitals, in their outermost electron shells. The titanium atom on the surface of the crystal has an empty orbital.
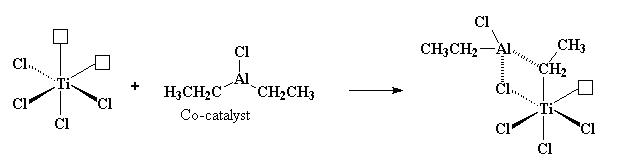
Al(C2H5)2Cl , the co-catalyst, donates one of its ethyl groups to the impoverished titanium, but in the process, one of the chlorines leaves. Therefore, there is still an empty orbital on the titanium.
The aluminium stays coordinated, though not covalently bonded, to the CH2 carbon atom of the ethyl group it just donated to the titanium. It also coordinates itself to one of the chlorine atoms adjacent to the titanium.
So then a vinyl monomer like propylene comes along. The two electrons in the π-system of the carbon-carbon double bond can be used to fill the empty orbital of the titanium. The propylene and the titanium form a complex.
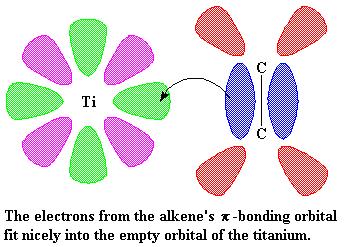
Alkene-metal complexes
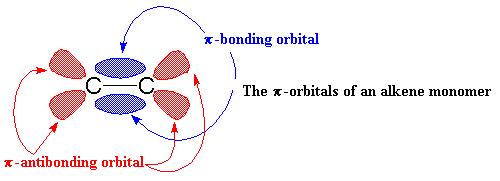
A carbon-carbon double bond, is made up of a σ bond and a π bond.
The π bond consists of two π- orbitals. One is the π-bonding orbital shown in blue and the other is the π-antibonding orbital, shown in red. The π-antibonding orbital is too high in energy, so majority of the time it stays empty.
The green lobes are empty orbital and the pink lobes are one of the filled orbitals. The empty orbital is going to share a of pair electrons with the alkene’s π-bonding orbital.
The Polymerization
Isotactic polymerization
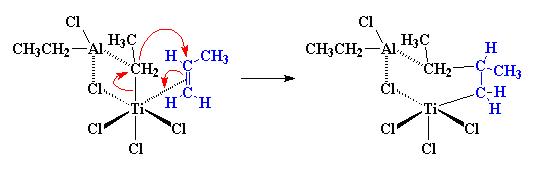
It is believed that the first electron pairs shift is that pair from the carbon-carbon π-bond that is complexed with the titanium. It’s going to shift to form simple titanium-carbon bond. Then the electrons from the bond between the carbon of the ethyl group and the titanium will shift to form a bond between the ethyl group and the methyl-substitued carbon of the propylene monomer.
Next a migration will occur. The atoms rearrange themselves to form a slightly different structure as below:
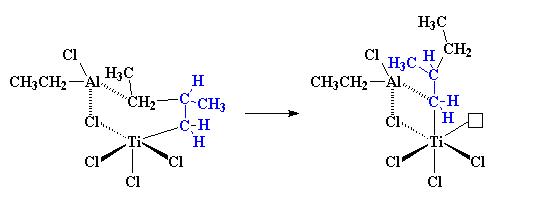
The aluminium is complexed with one of the carbon atoms from the propylene monomer. As it is shown, titanium is once again with an empty orbital.
So when another propylene molecule comes along, the whole process will starts all over and the polymerization continues and resulting:
This will react with more propylene molecules and the polymer chain extends. All the methyl groups on the growing polymer are on the same side of the chain. With this mechanism, isotactic polypropylene is produce.
Syndiotactic polymerization
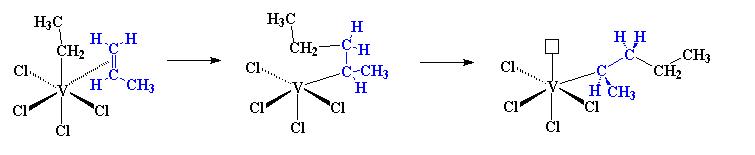
The mentioned catalyst system gives isotactic polymers, there are other systems tha can give syndiotactic polymers. The one that is focused on now is based on vanadium rather than titanium. That system is VCl4/Al(C2H5)2Cl. This complex will act similar to the titanium system. First the propylene will attack the vanadium and then the electrons will shift just like with titanium before and propylene is inserted between the metal and the ethyl group.
However, noted that when the second propylene adds to the chain, the chain changes position again. It’s back in the position where it started. The methyl groups of the first monomer in blue and the second monomer in red, they are on the opposite sides of the polymer chain. When the polymer chain is in one position the propylene monomer can only add so that the methyl group is on one side of the chain. When the chain is in the other position, propylene only adds the methyl group on the opposite side. Due to this switches positions with each propylene monomer added, the methyl groups are on alternating sides of the chain, producing a syndiotactic polymer.
Example of Titanium catalyst during polymerisation
Limitation
Ziegler-Natta doesn’t work for some kinds of monomers. For example, poly(vinylchoride) cannot be produced by Ziegler-Natta polymerization. This is because when the catalyst and the co-catalyst come together to form the initiating complex, radicals are being produced during the intermediate steps of the reaction, which will initiate free radical polymerization.
Reference
1. Potapov, A.G. / Bukatov, G.D. / Zakharov, V.A., Journal of Molecular Catalysis. A, Chemical, Mar 2006
2. http://www.hyle.org/journal/issues/5/cerruti.htm
3. http://www.pslc.ws/mactest/ziegler.htm
4. H. Hagen, J. Boersma and G. Koten, Chem. Soc. Rev., 2002, 31, 357