It:Warfarin
Warfarin
History
It was found that cows that grazed in fields planted with Sweet Clover would die from bleeds whose origin was unknown. The condition became known as Sweet clover disease. In 1921 it was found that rotting sweet clover was the cause of the disease and not the plant while it was growing in the field. It was not until 1941 that the molecule causing the bleeding was found within the mulch, when Karl Link found that dicoumarol had anticoagulant properties and was present within the decaying clover. From this he later synthesised the stronger blood thinner warfarin, to be used as rat poison.
Structure
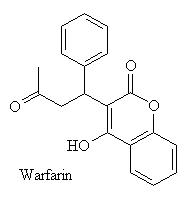
Warfarin contains a chiral centre, and therefore exists as two enantiomers, one with the benzene ring at the top of the diagram pointing out of the screen, and the other with it pointing down into the screen.

Biochemistry of anticoagulation
Warfarin gains it’s anticoagulation properties from its ability to disrupt the metabolism of vitamin K. Vitamin K is used in the synthesis of human clotting factors, the chemicals that instigate blood clotting, therefore if these are not present the blood will not start to clot. Warfarin does this by binding to the proteins needed to break down the vitamin K.
Conditions treated by Warfarin
Warfarin is used to treat all conditions caused by blood clots, eg strokes and DVTs. It is also used during certain surgeries to prevent clots forming during the operation. It has been used with other medicine to treat forms of lung cancer.
Side effects of treatment
Headache, upset stomach, diarrhea, fever, and skin rash.
Serious side effects
Unusual bleeding or bruising, black or bloody stools, blood in the urine, tiredness, unexplained fever, chills, sore throat, and stomach pain.
Resistance in rats
Over time rats have gained resistance to warfarin poisoning. Their blood clotting is effected, but it is merely slowed down rather than stopped all together. Their resistance is caused by their livers producing warfarin converting enzymes; these remove the warfarin from the blood and convert it into a metabolite of warfarin, which does not bind to vitamin K metabolic enzymes. Another possibility is that warfarin resistant rats have very slightly different vitamin K metabolic enzymes, that have a lower affinity for both vitamin K and warfarin, therefore these enzymes are inhibited much slower, allowing the body to remove the warfarin before it can have the desired effect.
References
http://www.chemsoc.org/ExemplarChem/entries/2003/imperial_Bhono/historical.html
http://www.aist.go.jp/RIODB/SDBS/cgi-bin/IMG.cgi?imgdir=ir&fname=NIDA29094&sdbsno=21930
http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1146900
http://www.nlm.nih.gov/medlineplus/druginfo/medmaster/a682277.html
http://www.nlm.nih.gov/medlineplus/druginfo/medmaster/a682277.html
http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=1179227&blobtype=pdf
