It:Vitamin E
| It:Vitamin E | ||||
|---|---|---|---|---|
| ||||
| General | ||||
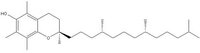
| Systematic name | (R)-2,5,7,8-tetramethyl-2-((4R,8R)-4,8,12-trimethyltridecyl)chroman-6-ol | |||
| Chemical Formula | C29H50O2 | |||
| CAS number | 59-02-9 | |||
| Properties | ||||
| Melting Point | 2.5-3.5°C | |||
| Boiling Point | 200-220°C | |||
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | ||||
VITAMIN E
Natural vitamin E has eight isomers; four of which are tocopherols and the other four tocotrienol, and they themselves have an alpha, beta, gamma and delta form (determined by the number of methyl groups on the chromanol ring). The most important Vitamin E is the alpha-tocopherol shown on the right. All isomers possess chromanol ring, which has a hydroxyl group that donates a hydrogen atom to reduce free radicals and hydrophobic side chain allowing penetration into organic membranes.
Importance/Functions of Vitamin E
Vitamin E like all vitamins is must have for growth and health, but in small amounts, obtained from your diet. Vitamin E is an antioxidant that protects living cells against oxidation reactions of free radicals (by-products of energy metabolism) that causes harmful effects such as cancer. It is also required for the proper function of nerves and muscles.
There are some conditions that might increase your need for vitamin E intake:
- Intestine disease
- Liver disease
- Pancreas disease
- Removed stomatch on operation
Sources of Vitamin E
Some examples of vitamin E rich sources:
- Wheat germ oil (215.4mg/100g)
- Sunflower oil (55.8mg/100g)
- Hazelnut (26.0mg/100g)
- Walnut oil (20.0mg/100g)
- Peanut oil (17.2mg/100g)
- Soybean oil (14.6mg/100g)
- Olive oil (12.0mg/100g)
Mechanism of Antioxidation
Vitamin E can capture the free radical, interrupting free radical chain reactions. The donation of the hydrogen from the free hydroxyl group on the aromatic ring to the free radical results in a relatively stable free radical form of the vitamin:

