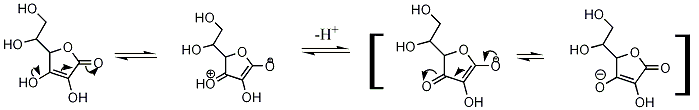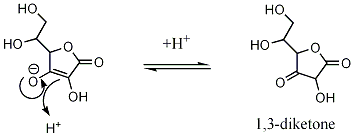It:Vitamin C
Introduction
The earliest story about Vitamin C happened in the seventheenth century. James Lind who was a ships surgeon in the British Royal Navy attempted to give scientific basis for the cause of scurvy. His test was conducted at sea in May 1747 and involved two groups of men. One group was provded with lemon juice in addition to their normal ration while the other was not. The results of the tests conclusively showed that lemons (in fact, the vitamin) prevented the disease.
Vitamin C (ascorbic acid) which is the most famous in all vitamins was the first vitamin to be discovered (1928), the first to be structurally characterized (1933), and the first to be synthesized in the laboratory (1933). It is essential in wound healing and in the formation of collagen, a protein important in the formation of healthy skin, tendos, bones, and supportive tissues. However, humans do not have the ability to make our own vitamin C and therefore the only way by which we can obtain vitamin C is through our diet.
| Ascorbic acid | |||
|---|---|---|---|
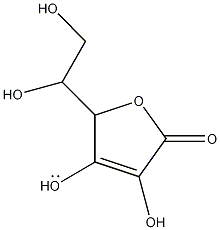
| |||
| 3D structure | |||
| Chemical name | '2-oxo-L-threo-hexono-1,4-lactone-2,3-enediol
or ( R )-3,4-dihydroxy-5-((S)-1,2-dihydoxyethyl)furan-2(5H)-one ' | ||
| CAS nummber | 50-81-7 | ||
| Chemical formula | C6H8O6 | ||
| Molecular mass | 176.13 g/mol | ||
| SMILES | OC1=C(C(O[C@@H]1[C@H](CO)O)=O)O | ||
| Density | 1.65 | ||
| Melting point | 190 -192 ºC(decomposes) | ||
Brief description
Vitamin C is an ascorbic acid which is only weakly acidic. The asorbic acid compound shows optical isomerisim producing two enantiomers of which only the L- enantiomer shows biological activity, its mirror image (the D- emantiomer) shows none . Vitamin C for commercial use is usually a mix of ascorbic acid , sodium ascorbate and sometimes various other ascorbates, sometimes they contain both the L and the D-enantiomer of the acid as it is harmless.
Use of vitamin C
• Antiscorbutic properties
-It prevents the onset of scurvy, a bleeding disease affecting those with a deficiency of fresh vegetables and citrus fruits in their diet.
• Reductant in photographic developer solutions and as a preservative. • Ascorbic acid and its sodium, potassium, and calcium salts are commonly used as antioxidant food additives.
Other possible uses of vitamin C (lacking scientific proofs)
• Prevent the common cold
• Prevent the cure infertility
• Inhibit the development of gastric and cancers.
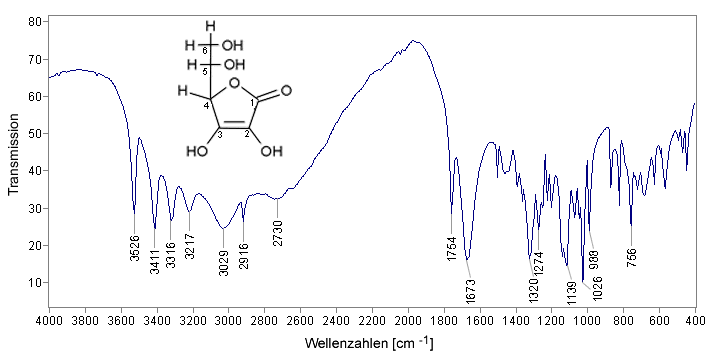
IR spectrum of Vitamin C
Acidity of Vitamin C
Tautomerism
Ascorbic acid can also interchange to two unstable diketone tautomers, 1,2-diketone and 1,3-diketone.
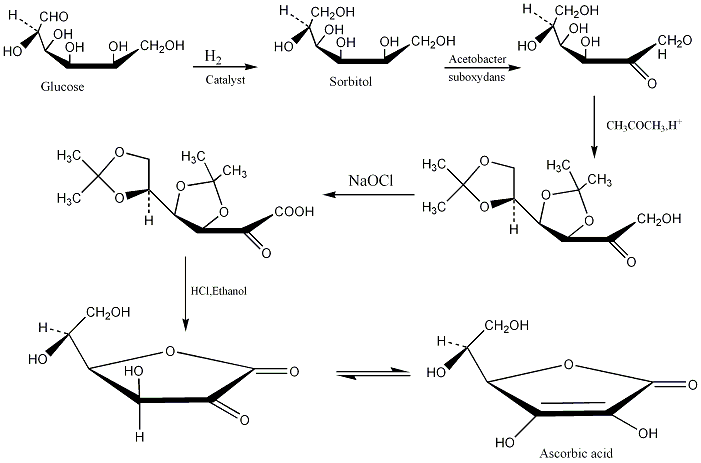
Synthesis
The Hoffmann-La Roche Company synthesizes ascorbic acid from glucose through the 5-step rout shown below:
Reference
Orangic Chemistry by John Mcmurry
http://en.wikipedia.org/wiki/Vitamin_C