It:Vanillin
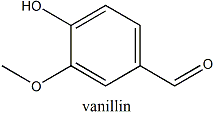
Vanillin
Natural vanillin (or 4-hydroxy-3-methoxybenzaldehyde) is the main component of oil of vanilla, the essential oil (the fragrance of a plant) obtained from a type of orchid called Vanilla fragrans or vanilla orchid. Its formula is C8H8O3 and its 3D structure is shown below:
Most essential oils are obtained by distilling the leaves or petals of the plant over steam but oil of vanilla is extracted from the dried, fermented seed pods.
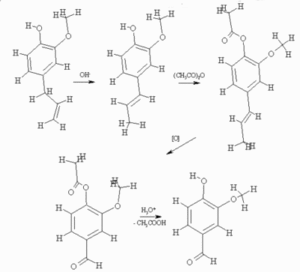
The majority of essentials oils are usually used in the perfume industry although some are also used in the food industry, particularly to flavour foods. Vanillin is used widely in both and because of this there is not enough natural supply to meet demand and so therefore has to be synthesised. This is mainly done by the oxidation of eugenol (or 2-methoxy-4-(2-propenyl)phenol) (which can be found in oil of bay found in bay leaves) which is shown below:
Synthesis Methods
Vanillin was first synthesized from Eugenol, up to 1920s. Following that, it was synthesized using waste materials from wood pulp processing, from a type of sufite liquor. Later on, due to environmental concerns, this method was disused and replaced by total synthesis from Guaiacol, a petrochemical raw material. The most important of these is the reaction by Rhodia involving the electrophilic aromatic substitution using Guaiacol and Glyoxylic acid, and doing an oxylative decarboxylation to convert it into Vanillin. The production cost of Vanillin using this method is about $15/kg. New methods of synthesis have been invented recently using biological molecules, but they are not cost efficient, costing up to $700/kg.
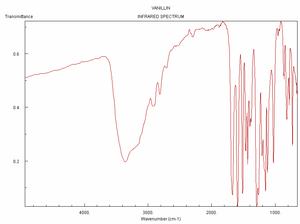
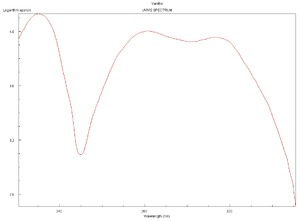
Spectra
Click on a spectrum to view