It:Tryptophan
Introduction
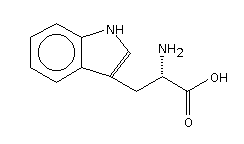
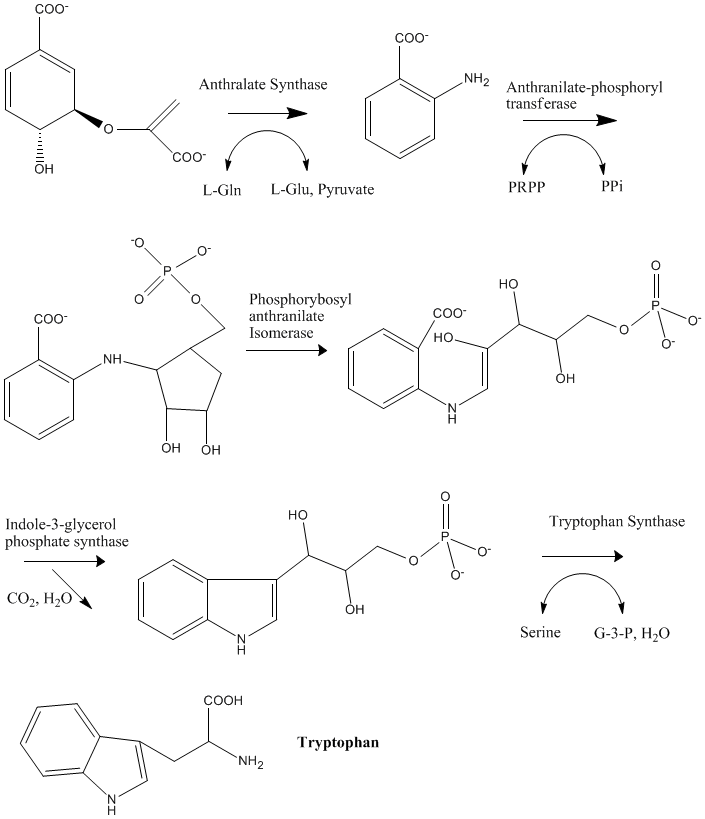
L-Tryptophan can be thought of as a natural anti-depressant. It is a naturally occuring amino acid that the body takes in through protein foods but can be lost through cooking. It is a precursor for the mood-controlling serotonin, which is a brain neurotransmitter linked with happiness. Tryptophan is one of eight amino acids that cannot be produced by animals. It is the least abundant of the essential amino acids, but it is also one of the most crucial, because of its involvment in the formation of serotonin.
3D Molecular Image
Medical Uses
Serotonin deficiency is often associated with depression and obesity, but simply adding it directly to the diet is not effective as it cannot pass the blood-brain barrier. This however can be done by tryptophan's chemical successor 5-hydroxytryptophan and thus an increased intake in tryptophan, which turns into 5-hydroxytryptophan in the body, can counteract the lack of serotonin. Since tryptophan can be cheaply produced and is not regulated as a drug, it is a better alternative to normal antidepressants.
Serotonin deficiency is also associated with obesity, as it has been shown that with excessive levels of sugars and carbohydrate intake as is common for obese people, serotonin is produced. Hence the intake of tryptophan can stimulate serotonin production and the lack of desire for sugar-rich foods.
Dangers previously associated with Tryptophan
In 1989 tryptophan supplements were banned as a result of a series of deaths which could be traced back to the intake of tryptophan. However the reason for these deaths was the use of inadequate purification of the substance and the fact that it had been synthesised using a certain type of genetically modified bacteria which produced toxins. No other methods of production produced these toxins and hence the ban has been lifted in several countries. However, tryptophan is still often used by many as an example against the use of genetically engineered products.