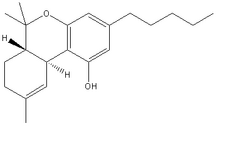
It:Tetrahydrocannabinol
Introduction
Cannabis has around 60 different psychoactive chemicals of which tetrahydrocannabinol (THC) is the main one that acts on the nervous system. It is known as the source of the marijuana drug. It was first isolated by Raphael Mechoulam and Yechiel Gaoni from Weizmann Institute in Rehovot in 1964.
Properties
THC has a good solubility in organic solvents, e.g. hexane or ethanol, but not in water. If the glassy solid is warmed it becomes viscous and sticky.
Isomerisation
There are two isomers of THC found in marijuana: Δ8- and Δ9-tetrahydrocannabinol. Δ8-THC compromises around 10% of total tetrahydrocannabinol, which might be formed by isomerisation since it has similar pharmacological activity to Δ9-THC.
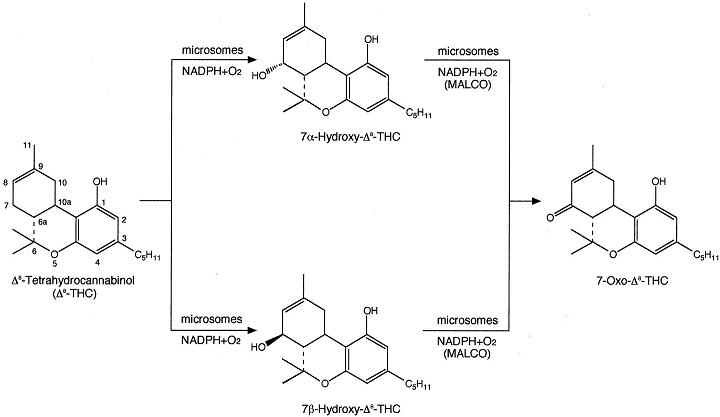
The most reactive sites for Δ8- THC are at C-7 and for Δ9-THC at C-8; and C-11 for both isomers. Secondary alcohols such as hydroxysteroids are oxidized to the equivalent ketones by the enzyme dehydrogenases in cytosol and microsomes. However, it was discovered that microsomal alcohol oxygenase (MALCO) can also oxidise 7 - and 7 -hydroxy- 8-THC to 7-oxo- 8-THC. In humans it is catalysed by CYP3A4.
Synthesis
In this article three active isomers are considered, which differ in the bonding of the left hand ring:
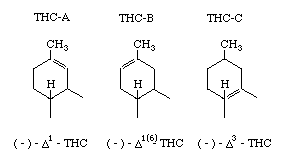
On the picture THC-A is shown as Δ1-THC, which is same as Δ9-THC since the numbering of the ring was different when this was discovered. Tetrahydrocannabinol A and B are found in hashish, while C is a synthetic one. The potency of these three isomers is similar, but by changing the alkyl group of the right hand ring an increase in effectiveness has been shown. The synthesis of all three of them is based on the condensation of olivetol with a compound that forms the alkyl group for increase activity. An example of preparing THC-V is shown below.
Effects in Human
Tetrahydrocannabinol binds to the cannabinoid receptor CB1, which is located in the brain. Even at low doses it has analgesic effects.
Its effects are:
• Relaxation • Euphoria • Altered space-time perception • Alteration of visual, auditory and olfactory senses • Disorientation • Fatigue • Appetite simulation • Anti-emetic effects
Due to these effects Δ9-THC is used in the medicine for several causes:
1. As an antiemetic to control nausea and vomiting associated with cancer chemotherapy.
2. As an antiemetic to control nausea and vomiting associated with the use of drugs for controlling the spread of the
AIDS virus; without which patients could regurgitate the nausea inducing drugs before being properly absorbed by the body.
3. To slow down or reverse the weight reduction syndrome of AIDS.
4. To control seizures in patients suffering from epilepsy.
5. To reduce pressure within the eyes associated with glaucoma.
6. To alleviate muscle spasms associated with multiple sclerosis.
7. To alleviate pain and muscle spasms for paraplegics and quadriplegics.
Degradation of THC
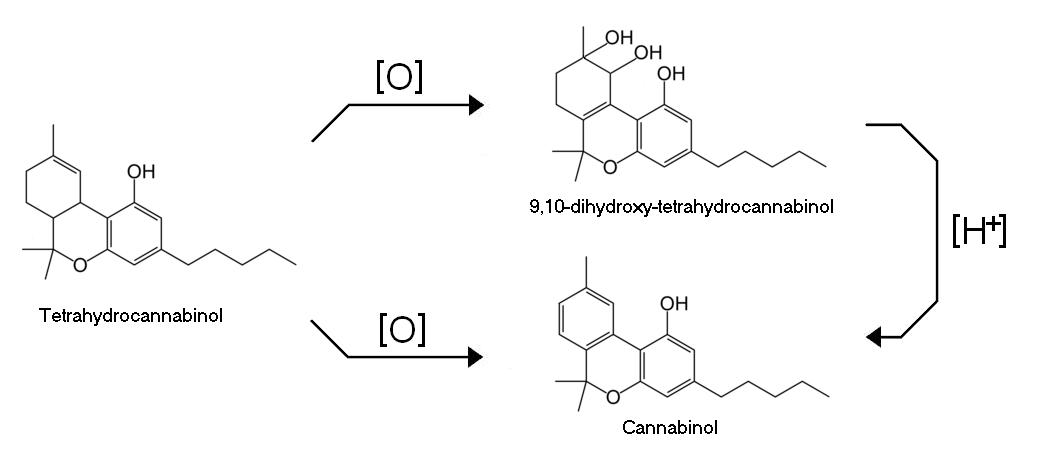
Tetrahydrocannabinol undergoes oxidation to either cannabinol or 9,10-dihydroxy-tetrahydrocannabinol. Cannabinol (CBN) is also the natural degradation (oxidative) product of THC. Fresh samples of marijuana contain very little CBN but curing, or poor storage, over long periods can cause much of the THC to be oxidized to CBN. It is also possible to reduce 9,10-dihydroxy-tetrahydrocannabinol in the presence of acid to CBN.
Pure forms of CBN have at most 10 percent of the psychoactivity of THC. CBN seems to potentiate THC's disorienting qualities. One may feel more dizzy or drugged or generally messed up but not necessarily higher. In fact, with a high proportion of CBN, a 'high' may start well but feels as if it never quite reaches its peak, and when coming down one feels tired or sleepy.
Reference
• http://dmd.aspetjournals.org/cgi/content/full/29/11/1485#F1
• http://en.wikipedia.org/wiki/Tetrahydrocannabinol
• http://designer-drugs.com/pte/12.162.180.114/dcd/chemistry/thc/index.html
• http://www.a1b2c3.com/drugs/mj028.htm
Cannabis. XV. Preparation and Stability of d9-Tetrahydrocannabinol-B-Cyclodextrin inclusion complex Y. Shoyama, S. Morimoto, and I. Nishioka. Journal of Natural Products, Vol 46, No.5, pp. 633-637, Sept-Oct 1983