It:Tamoxifen
Tamoxifen
Structures and 3D Image
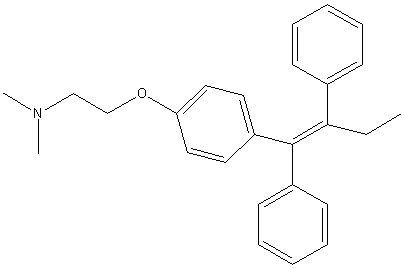
Structure of Tamoxifen 1,2
Chemical Information
| IUPAC name | (Z) 2-[4-(1,2-diphenylbut-1-enyl)phenoxy]-N,N-dimethyl-ethanamine. |
|---|---|
| Chemical Formula | C26H29NO |
| Molecular Weight | 371.52 g mol-1 |
| Melting Point | 96-98oC |
| Type of Substance | White, Odourless Crystals |
What is Tamoxifen Used For?
Tamoxifen is currently the most popular drug used for the treatment of breast cancer in women. Tamoxifen was discovered by AstraZeneca (formally known as ICI Pharmaceuticals) and is commercially known as Nolvadex, Istubal and Valodex. It is taken as a tablet
It has also been found that Tamoxifen can be used to treat other ailments, such as osteoporosis and gynecomastia in men.
Tamoxifen is occasionally used to improve the rare condition known as retroperitoneal fibrosis (or Ormond's disease), although the exact mechanism of this reaction is still unknown. This condition involves an excess of fibrous tissue and scar tissue in the part of the body which contains the kidneys, aorta, renal tract and various other structures. Symptoms of the condition improved within weeks, and within months signs of inflammation decreased and the scar tissue shrunk.
How Does Tamoxifen Work?
The primary function of Tamoxifen is as an anti-oestrogen drug, which means it reduces or stops the action of oestrogen.3
Breast cancer is hormone-related and relies on the supply of sex hormones, particularly oestrogen, to grow. Cancers with oestrogen receptors on the surface of their cells are called `oestrogen receptor positive' (ER positive). Oestrogen can fit directly into the active site on these receptors and causes the cells to divide and the tumour to grow (see Figure 1).4
Tamoxifen has a similar structure to oestrogen and works by imitating the action of oestrogen and fits into the active sites of the receptors. However, tamoxifen does not cause the cells to divide, but remains in place and prevents the oestrogen from getting to the cancer cells, therefore they either grow more slowly or stop growing altogether (see Figure 2).
How is Tamoxifen made?
Synthesis of Tamoxifen via a carbometalation of alkynylsilanes
Step 1
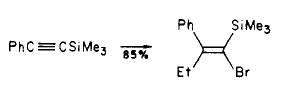
Step 2
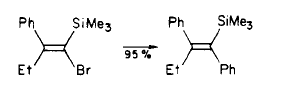
Step 3

Step 4
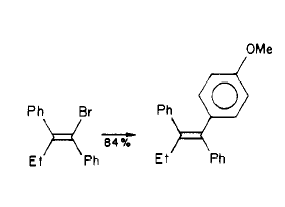
Step 5
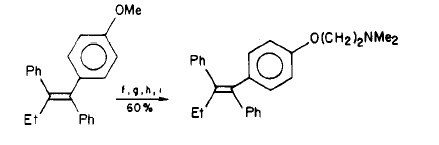
Side Effects of using Tamoxifen
The most common side-effects of taking Tamoxifen in pre-menopausal women are:
- nausea
- hot flushes and sweats
some less common side-effects include
- headaches
- tiredness
- dizziness.
For post-menopausal women who take Tamoxifen over a long period of time, there is a slightly increased risk of developing endometrial cancer, however, if detected early, treatment for this is usually successful and the benefits of taking Tamoxifen in treating breast-cancer far outweigh this small risk.5
References
- 1 - MDS Crossfire Commander Beilstein: Structure
- 2 - Conquest (Cambridge Crystal Structure Database): 3D image
- 3 - www.cancer-info.com/tamoxifen.htm
- 4 - www.fareston.com/d_living/d3.html
- 5 - www.cancerbackup.org.uk/Treatments/Hormonaltherapies/Individualhormonaltherapies/Tamoxifen
- 6 - R. Bryan Miller, Mohammed I. Al-Hassan Stereospecific Synthesis of (Z) - Tamoxifen via Carbometalation of Alkynylsilanes J. Org. Chem. 1985 50, 2121-2123
- 7 - http://www.annals.org/cgi/content/full/144/2/I-51
- 8 - http://en.wikipedia.org/wiki/Retroperitoneal_fibrosis
