It:Tamiflu
| Tamiflu | |
|---|---|
| |
| IUPAC Systematic name | |
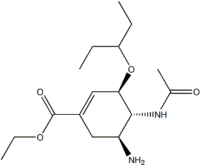
| (3R,4R,5S)-4-acetylamino-5-amino-3-
(1-ethylpropoxy)-1-cyclohexene- 1-carboxylic acid ethyl ester | |
| Other name | |
| Tamiflu | |
| Indentifiers | |
| ATC Code | J05AH02 |
| CAS number | 196618-13-0 |
| PubChem (CID) | 65028 |
| SMILES | [C@@H(OC(CC)CC)
[C@H](NC(C)=O)[C@@H](N)C1)OCC' 'O=C(C1=C[C@@H](OC(CC)CC) [C@H](NC(C)=O)[C@@H](N)C1)OCC'] |
| Chemical Data | |
| Molecular formula | C16H28N2O4 |
| Molar mass | 312.4 g/mol g/mol |
| Pharmacokinetic Data | |
| Bioavailability | 75% |
| Protein Binding | None |
| Metabolism | hepatic, to GS4071 |
| Half life | 6-10hours |
| Excretion | renal (GS4071) |
| Therapeutic considerations | |
| Pregnancy cat. | B1, C |
| Legal status | - |
| Routes | Oral |
Introduction
Tamiflu is an antiviral currently used to treat flu pandemic victims. It acts by inhibiting neuraminidase that lives on the flu virus cells. Tamiflu was developed by Gilead Sciences, and it is currently marketed by Roche.
Structure
Synthesis
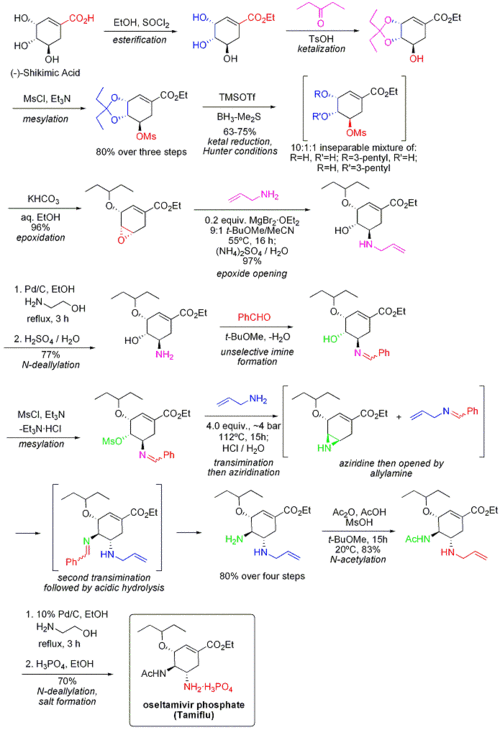
The synthesis of Tamiflu uses shikimic acid which is extracted from the pods of star anise. The commercial synthesis of Tamiflu uses a complex 10 step synthesis with some steps using explosive azide. This limits the speed at which synthesis may be carried out. The process is extremely long, approximately 6-8 months to complete. To make things worse, 30kg of star anise only produces 1kg of shikimic acid. Hence, in order to be able to produce the quantities of Tamiflu required to fight a flu pandemic, either another source of shikimic acid must be found or another synthesis devised.
Image taken from http://en.wikipedia.org/wiki/Oseltamivir
Mode of action
Tamiflu binds to the neuraminidase on the surface of the virus, preventing it from invading the cell. This only inhibits the virus and not destroy it, thus at a certain point in time the drug will be useless as the infection would be too great to be inhibited effectively. Neuraminidase is important to the virus to both enter and exit the cell, thus by binding to it the infection is stopped as the virus can no longer leave and infect other cells.
References
- http://en.wikipedia.org/wiki/Oseltamivir
- http://www-jmg.ch.cam.ac.uk/data/molecules/misc/tamiflu.html
- http://www.chm.bris.ac.uk/webprojects2006/Campbell/index.htm
- http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=65028
- http://www.tamiflu.com
- http://www.journalresponse.com/search.php?query=Oseltamivir
- J. Org. Chem. 1998, 63, 4545-4550. Synthesis of Tamiflu.