It:Scopolamine
Scopolamine
Abstract
As is common knowledge, scopolamine (systematic name (alpha)-(hydroxymethyl) benzeneacetic acid 9-methyl-3-oxa-9-azatricyclo [3.3.1.0 2,4] non-7-yl ester) was used during the Second World War as a 'truth drug' by the German Military and Intelligence (as many viewers and fans of 'The Guns of Navarone' would know). However, serving in this role, its success was limited, and scopolamine had (and indeed has) several other roles, neurological and otherwise, in human physiology and pharmacology. While it was used as a depressant (being an anticholigenic drug), it worked to mollify patients thanks to its sedative capabilities, thus making it easier to obtain 'blind' answers to question.
However, scopolamine is one of the drugs that is not technically synthesized chemically. Like insulin, scopolamine is produced (if required to be on a mass scale) by splicing the relevant gene responsible for its (enzyme) production onto the RNA sequence of a bacterium, usually of the E. coli family, or a derivative of it. Also, even then it needs the precursor to it to be present or produced: hyoscyamine.
In actuality, scopolamine is not all that common in the higher plants, one of the few plants capable of making it being Hyoscyamus niger. In the case of H. niger the drug is produced in the branch roots. The reason behind the lack of frequency of the presence of scopolamine throughout the Solonaceae family of plants, is the lack of a particular gene. This gene is responsible for the production of the enzyme which catalyses the synthesis of scopolamine from hyoscyamine: hyoscyamine 6 [beta]-hydroxylase (often abbreviated to H6H).
Medically speaking though, the drug is rarely taken as its naturally occurring form. As with the majority of other drugs, it is taken as a salt analogue: scopolamine hydrochloride; or -methyl nitrate. Moreover, there are other analogues of scopolamine, such as atropine, though it is considered that the former has a greater central nervous systemic effect (hence its use in the treatment of motion sickness).
Chemical Structure & Properties
Scopolamine has the structure as below:
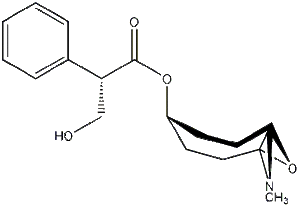
| Chemical & Biological Properties | |||
| Chemical Formula | C17H21NO4 | ||
| Melting Point | 381.2-390.2°C (as nitrate), 55°C (as hydrobromide trihydrate) | ||
| Principle Indications | excessive salivation, IBS, motion sickness | ||
| Additional | colicky abdominal pain, sialorrhoea | ||
| Mechanism | interference of ACh* in nerve impulses | ||
| Biological Half-Life | approx. 9.5 hours in normal adult | ||
| Solubility | highly soluble in both organic & inorganic solvents | ||
| 3D Structure | |||
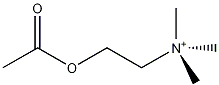
- ACh = acetylcholine, see below
Biological Mechanism
Scopolamine works by interfering with one of two sets of acetylcholine receptor sites which are the nicotinic (nAChR) and muscarinic (mAChR). The drug prevents the acetylcholine from binding with the latter (and nicotine induces a hypersensitivity at the former). This Is done by the scopolamine itself binding to the receptor in the place of the ACh, thereby blocking it. Having done so, being attached, it creates no inducive effect for the nerve impulse to proceed, hence, the sedative effect, as chemical signals back and forth between brain and skeletal muscles are slowed down and/or prevented. As it acts more centrally to the spinal column and brain, it therefore dulls pain to the thorax and abdomen (the nerve arcs to the vital organs and those in close proximity being much shorter), thus its effectiveness in treating motion sickness and IBS (irritable bowel syndrome).
Side effects
Scopolamine has a lot of nerological side effects, and overdose on thedrug can lead to dillusion, delirium, paralysis, stupour and death. It also causes the pupils to dilate, and paralysis of the eye muscles in medium doses. The drug is also known to cause anisocoria, which is an effect characterised by an unequal size of the pupils and nausea. For this reason the effects of the drug can be confused with brain cancer, as these effects (nausea, and anisocoria) are also seen in patients with brain tumours.
Uses
Scopolamine was one of the drugs investigated in the 1950's for its 'truth drug' or mind control properties by the CIA and the British government. The investigations began on the back of intelligence obtained during the Cold War suggesting that the Soviets had themselves obtained mind control capabilities. Other drugs tested by the intelligence agencies included but were not limited to LSD, mescaline, and quinuclidinyl benzyilate other chemical psycological and biological methods of mind control were also explored including for example sleep depravation.The test subjects would be given the drugs, then interrogated and their answeres recorded and analysed. Subjects would often be given the drugs without their knowledge and their reactions would be monitored. Scopolamine was abandoned as a possible truth drug because its hallucinogenic side effects meant that the answers obtained from subjects under the influence of the drug were were prone to distortion.
The drug has also found use as a 'date rape' drug, and also as an aid to robbery. In these cases the drug is usually administered to the victim by drugging the persons drink. It is used for this purpose because of its ability to cause amniesia in the victim, meaning that they forget events that happen prior to taking the drug.
As mentioned above the drug is also used in the treatment of motion sickness, and has become popular among divers for its use in the treatment of sea-sickness. It must though be taken with caution by divers as it has been found to cause pain in the eyes when divers reach depths of about 50 feet. This pain usually subsides if the diver rises to 40 feet or above.
References
Extended thanks to the following locations and parties for the use of their material:
Species-Dependent Expression of the H6H Gene in the Pericycle Plant Physiology (Vol 105, 483-490): Molecular Biology & Gene Regulation - T. Kanegae, H. Kajiya, Y. Amano, T. Hashimoto and Y. Yamada
Scopolamine(APRD00616) DrugBank
Scopolamine Drug Information Drug Information Online (Prescription Drug Information for Consumers & Professionals)
Information on Scopolamine use in illegal activities
also see Wikipedia's Acetylcholine
