It:Salmeterol
Introduction to Salmeterol
The drug Salmeterol C25H37NO4 is a beta agonist, and is prescribed by doctors under the name Serevent available as an inhaler for asthma sufferers. It is mainly used when asthma has not been controlled in extreme circumstances and can be used along side a beta agonist like salbutamol. These beta agonists are used to relieve worsening asthma and so should be used along side corticosteroid such as beclomethasone.
Some Concerns
Although the benefit of salmeterol of actually lasting for 12 hours whereas salbutamol lasts up to 4 hours was a breakthrough for chemical company Glaxo Smith Kline, there have been links that the drug has long term side effects of worsening asthma and its attacks leading to hospitalisation or even death. Patients should only be prescribed the drug if the asthma is not being controlled with the current corticosteroid and those with severe asthma. It has been believed that many sufferers that have not been taught to control their asthma have been taking the drug unnecessarily, and unknowingly increasing the risk of more severe attacks leading to death.
How it Works
When salmeterol is inhaled it relaxes and smoothens the bronchial walls allowing the tubes to expand so more air can be carried. Salmeterol’s long, lipophilic side chain binds to sites near beta(2)-receptors in the lungs and on bronchial muscles. This allows the active portion of the molecule to stay at the receptor site in the lungs, continually binding and releasing. This is why it last longer than the other agonist salbutamol. The beta(2)-receptor stimulation in the lung causes relaxation of bronchial muscles i.e. it causes bronchodilation, and increases bronchial airflow.
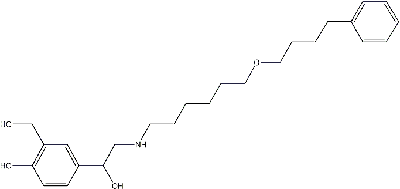
Structure of Salmeterol
Properties of Salmeterol
| Properites | ||
| IUPAC Systematic Name | 2-(hydroxymethyl)-4- [1-hydroxy-2-[6-(4-phenylbutoxy) hexylamino]ethyl]-phenol | |
| Chemical Name | Salmeterol | |
| Molecular formula | C25H37NO4 | |
| Molecular Mass | 415.566 gmol-1 | |
| Melting Point | 75.5 - 76.5oC | |
| Solubility | Sparingly soluble in H2O | |
| Type of Substance | Beta Agonist | |
| CAS Registry Number | 89365-50-4 | |
| SMILES String | OCC1=C(O)C=CC(C(CNCCCCCCOCCCCC2=CC=CC=C2)O)=C1 | |
Synthesis of Salmeterol
There are 7 steps involved in the preparation of salmeterol
Synthetic Route
- The synthesis begins by condensing the brokomketone in(i)
- A butoxycarbonyl group is then removed in (ii)
- This was reduced to give the resulting protected amino alcohol in (iii)
- Treatment with DMF and sodium hydride gave the oxazolidinone product of (iv)
- This intermediate is a stable crystalline solid and is then alkylated with the appropriate elctrophile in (v)
- Treatment with 2 equivalents of patassium trimethylsilanolate in reflux of tetrahydrofuran gives the amino alcohol of (vi)
- This is then deprotected to give the final product salmeterol (vii)
References
1. http://pubchem.com/chemical/Salmeterol_xinafoate.html