It:Quinine
Overview
Quinine, (2-ethenyl-4-azabicyclo[2.2.2]oct-5-yl)- (6-methoxyquinolin-4-yl)-methanol has historically been used orally as prophylactic and treatment against Malaria as well as a muscle relaxant, analgesic and an anti-imflammitory drug. Although now superceded by more modern pharmaceuticals such as Mefloquine hydrochloride (trade name; Lariam), it is still produced one a small scale as a drug as well as a flavouring to the soft drink and mixer, 'tonic water' giving the drink it's distinctive better taste.
Structure
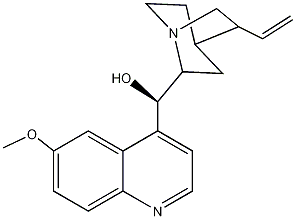 |
| ||
|---|---|---|---|
| 2D Structure | 3D Structure |
Description
White, crystalline alkaloid with a bitter taste.
| Template:Chemical header| |
|---|
| Template:Chemical header| Chemical Data | |
|---|---|
| Systematic (IUPAC) name | (2-ethenyl-4-azabicyclo[2.2.2]oct-5-yl)
-(6-methoxyquinolin-4-yl)-methanol |
| Molecular formula | C20H24N2O2 |
| Molecular mass | 324.417 g/mol |
| Melting point | 177 °C |
| CAS number | 150-95-0 |
Uses of Quinine: A Very Brief History

An extraction from the bark of the Cinchona tree (found mainly in South America) had been used to treat fever since before the 1600s by the Peruvian Indians before European explorers found that it was also an effective treatment for Malaria. During 1820, on it's introduction in Europe as a treatment for Malaria sufferers that quinine, the active compound in the bark, was first isolated and named quinine. The early cure for malaria in Europe was in fact a healthy dose of gin and tonic.
Use and supply of quinine was continued until 1941, when Japan invaded Java (at that point supplier of 95% of the world's Cinchona bark) and prevented its export. Alternative malaria treatments were hastily developed (chloroquine and mefloquine for example) for treatment of soldiers during the war. In 1957 a global campaign was launched using these new drugs aiming to totally eradicate the malaria parasite. However, it was soon realised that the parasite developed a resistance and drugs became less and less effective. Consequently, interest in quinine has risen considerably since with the aim of developing a more effective treatment.
Quinine and Malaria
As a consequence of the ongoing global threat of malaria, research into cures and ways to prevent the disease spreading is also a continuous process. However, as the bacteria gains resistance to man-made drugs, interest is returning to the use of quinine as an effective cure. Around 400 million people suffer from infection every year. Those particularly effected are those in tropical and subtropical regions. The disease is caused by protozoan parasites of the genus Plasmodium. The prasite is primarily carried by female mosquitoes of the Anopheles genus. Young mosquitoes first ingest the malaria parasite by feeding on an the blood of previuosly infected humans. The parasite is then carried in the saliva glands, and is passed on during the next meal. The symptoms of malaria are caused when the bacteria attack the red blood cells.
Quinine is an effective treatment still because if affects the way that the bacteria interacts with haemoglobin in the blood.
Mechanism of Action
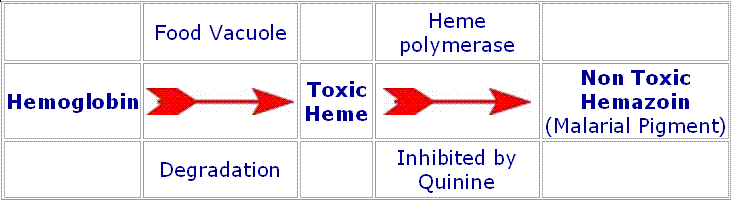
It is understood that the quinine is effective due to the fact that it is toxic to the malaria parasite, specifically by interfering with the parasite's ability to break down and digest hemoglobin. Consequently, the overall effect is to starve the parasite or alternatively cause the build-up of toxic levels of partially degraded hemoglobin in the parasite. Both have the effect of preventing the parasite from causing problems in the haemoglobin.
Synthesis of Quinine
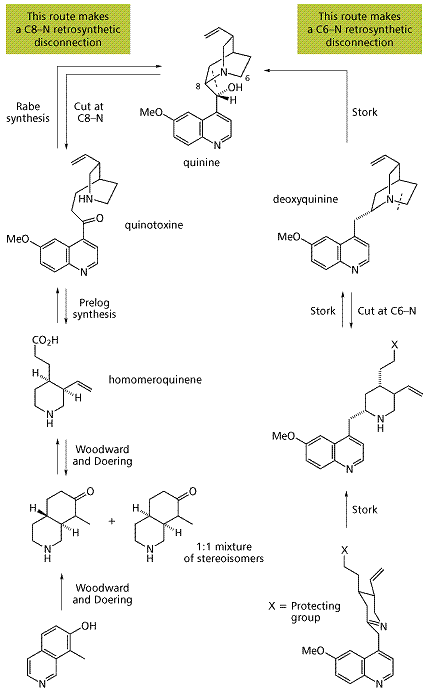
Although Woodward and Doering are credited with having found the first synthesis of quinine in 1944, they actually refined a method first discovered in 1918 by Paul Rabe. His process was less effective as it did not consider the stereochemistry of the final product, and as a result a racemic mixture of two enatiomers was produced. It was in 1960 that a chemist named stork analysed the retrosynthesis of quinine and devised a new method of production. However, it can be seen rom the reaction scheme that boh processes are long and complicated. Neither are used to a huge extent comercially, and whenever possible quinine is still extracted from cinchona bark.
References
http://www.nlm.nih.gov/medlineplus/druginfo/medmaster/a682322.html
http://www.rxlist.com/cgi/generic3/quinine.htm
http://redpoll.pharmacy.ualberta.ca/drugbank/cgi-bin/getCard.cgi?CARD=APRD00563.txt
