It:Propanil
| Structure of Propanil | ||||
|---|---|---|---|---|
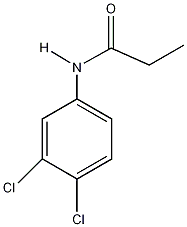
| ||||
| 3D structure | ||||
| CA Index Name | Propanil,
3,4-dichloropropionalide | |||
| Commercial Names | Bay 30130
Cekupropanil Dropaven Surcopur | |||
| Molecular formula | C9H9Cl2NO | |||
| SMILES | ClC1=CC=C(NC(CC)=O)C=C1Cl | |||
| Molar mass | 218.08 g/mol | |||
| CAS number | 709-98-8 | |||
| Appearance | white to brown crystalline solid | |||
| Odour | mildly offensive | |||
| Properties | ||||
| Density and Phase | 1.054g/cm3, solid | |||
| Solubility in water | 225 ppm at 25°C | |||
| Melting point | 91-93 °C | |||
| Flash Point | 100.0°C | |||
| Vapor Pressure | 2.6x10-7 mbar | |||
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | ||||
What is Propanil?
Propanil is an anilide used as a herbicide in a number of economically important crops, such as rice, potato and corn, against broad-leaved and grass weeds. It is sold as emulsifiable concentrates, liquid and dry flowable, low volume and ultra low volume formulations. Early weed control removes competition, conserves moisture and contributes to increased yields. Propanil formulations may be used on dry planted, hydroponic, or transplanted crops.
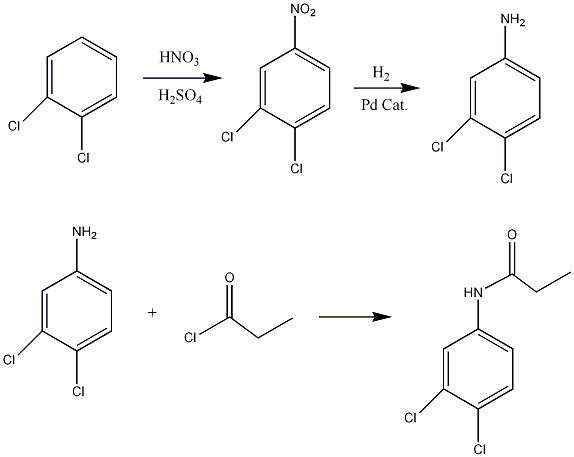
Synthesis
Spectra
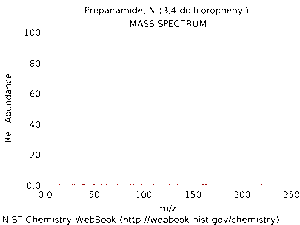
Below are spectra of propanil. The first is an IR spectrum of propanil in its gas phase. The second is a mass spectrum of propanil. You can clearly see the parent molecular ion peak is positioned close to the recorded molecular mass of propanil - 218.08amu. There are two chlorine atoms in this compound, and peaks due to the different isotopes of chlorine, namely Cl35 and Cl37, are responsible for the lack of a clear single parent molecular ion peak. The recorded molecular mass of propanil takes into the account the differing masses of the 2 isotopes of chlorine and their relative proportions (approximately 75% 35Cl and 25% 37Cl).
References
- Stuart Warren, Organic Synthesis - The Disconnection Approach, pp27-28
- http://www.propanil.com/
- Extoxnet Page
- Electrochemical and Spectroscopic Studies of the Oxidation Mechanism of the Herbicide Propanil
- NIST Mass Spec Data Center, S.E. Stein, director