It:Polyurethane
Introduction
Polyurethane is any polymer with urethane linkage. It has many applications due to a wide variety of properties it possess, for examples, furniture cushioning, mattresses, textiles, refrigerated appliances, building blocks with integrated insulation, bonding foam, casting and surfacing, etc.
Chemistry of Polyurethane
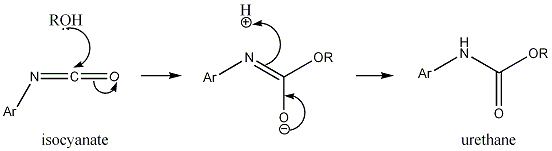
A simple urethane is formed when nucleophilic attack of alcohol on isocyanates. Urethanes are hybrids between carbonates and ureas – half-esters and half-amides of carbonic acid.
The nucleophilic reaction of isocyanate and alcohol gives a simple urethane as shown below:
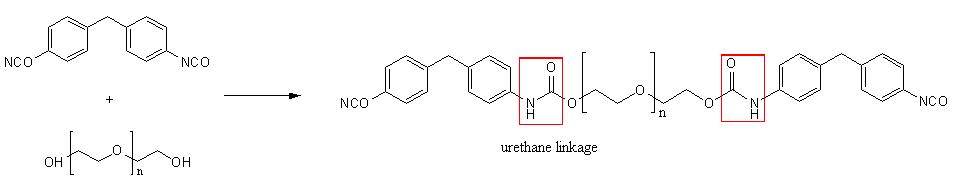
A prepolymer formed when a diisocyanate with diol. A long chain of polyurethane is formed when diisocyanates and polyols are reacted.
Reaction of diisocyanate and diol showing the urethane linkage:
3D structure of a simple copolymer of urethane:
Solid Polyurethane Elastomer
There is a wide variety of polyurethanes is being synthesised, but elastomer is one type of some important polymers from polyurethane. Polyurethane elastomers are rubber-like materials that can be created with a wide variety of properties and molded into almost any shape.
Properties
The properties of polyurethane varied with the types of polyols, diisocyanates used in the reaction. However, they all have certain characteristic properties in common. Polyurethanes have a high wear resistant to solvents and environment degradation; they also exhibit high elasticity within the different hardness ranges.
The other factors affecting the properties of the polyurethane are the processes of manufacturing polyurethane:
- Hot cure systems - This method results in linear sequences which exhibits a relatively rigid geometry.
- Cold cure systems - A three-dimensional network of low crosslink density polyurethane is formed from the reaction.
- Reactive spray coatings
General properties of polyurethane elastomers:
- Mechanical wear resistance
- Resistance to light, air, ozone and ultraviolet radiation (especially polyester-based materials)
- Absence of extractable ingredients such as plasticizers
- Low degree of swelling in mineral oils and fats
- Good notched impact resistance
Production of Solid Polyurethane Materials
Different methods can be used to mold solid polyurethanes. Castable polyurethanes are produced by pouring the blended liquid raw materials into a mold. Mixing and pouring these raw materials can be done manually or by using casting equipment.
Thermoplastic polyurethanes can be produced by injection molding, extruding or calendaring. By molding or “open heating” of rubber-like PU mixtures, production processes typically used in the rubber industry can be employed.
PUR Cast Systems
The oldest method of producing molded solid polyurethane parts is casting it into open molds. The liquid or molten components, which contain reactive NCO and OH or NH2 groups, are thoroughly mixed together and poured into open molds. It is essential to control material ratios and production conditions. Because further reaction will occurs in the mold as the mass solidifies. Hot or cold cure systems can be chosen according to production methods. Different method will determine the various chemical and physical properties desired in the end products. The physical properties of parts produced by the hot cure method are higher than those produced by room temperature cure. The majority of polyol components of hot-cast systems is based on polyester diols or polytetreamethylene glycols. Most cold cure systems use di- or tri-functional polypropylene glycol polyethers.
Hot cure systems: Production and Processing
Isocyanate-terminated MDI (4, 4’ diphenylmethane diisocyanate) or NDI (naphthalene diisocyanate) prepolymers are frequently prepared by the PU molders, so they can meet the manifold requirements in the different applications by individual adjustment of the formulation.
For hand mixing of the prepolymer, the polyol is heated, dewatered and placed in a reaction vessel. Liquid isocyanate is then added in one shot, generally in a molar excess. Since NDI cannot be added as a liquid to the polyol because of its high melting point (127°C), overheating of the prepolymer is prevented by heterogeneous reaction. This method of prepolymer formation is frequently used for the production of high monomer containing quasi-prepolymers of limited storage stability.
The mix ratio for the two components is determined by the properties (hardness) desired in the finished product.
Polyurethane Cold Cure System
Cold cure systems are mainly comprised of poly(oxypropylene)glycol or poly(oxyethylene-oxypropylene)glycol mixed ethers, sometimes from liquid polyesters or hydroxyl containing natural materials (castor oil). They are either processed by the prepolymer technique or the one-shot process. There is a slight difference between these two processes, because the prepolymer contains different amounts of monomeric isocyanates. However, in both processes, a mixture of long and short chain difunctional polyols, or partially branched polyols usually containing fillers, is used as the second component. This wide range of production processes leads to different properties in the finished product.
Comparing to the hot cure system, cold cure systems use prepolymers prepared on a large scale in a batch process. This large scale production will guarantee the required specified properties by accurate process control.
Cold cure systems can be done by hand mixing as well as by continuous or intermittent machine production. There are no basic differences between using a liquid diisocyanate and a prepolymer in a one shot method.
References
1. "Polyurethane Handbook 2nd Edition", by Gunter Oertel;New York: Hanswer, 1993
