It:Polydimethylsiloxane
POLYDIMETHYLSILOXANE
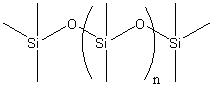
Polydimethylsiloxane is based on the repeat unit [(CH3)2SiO], is the most common silicon-based polymer industrially, with wide range of applications, e.g. adhesives, surfactants, lubricants, sealants etc. They are known mostly because of their special flow and deformation properties. The overall reaction for the synthesis of polydimethylsiloxane is:
Names, Structures and Properties
|
|
|---|
| Identity | |
|---|---|
| Synonyms | PDMS, Dimethylpolysiloxane, Dimethicone |
| Chemical Formula | (CH3)3SiO[SiO(CH3)2]nSi(CH3) |
| CAS Number | 63148-62-9 |
General Properties
- Resistance to heat, chemical attack, water and air (due to strong Si-C, C-H and Si-O bonds)
- Low toxicity
- Non-flammable
- Low surface tension
- High permeability
- High compressibility
- Viscoelastic (at high temperatures or long flow times it acts like a viscous liquid and at low temperatures or short flow times it acts like an elastic solid)
Physical Properties of Linear Polydimethylsiloxane
|
|---|
a M = Me3SiO1/2; D = Me2SiO.
Flexibility and Permeability
Flexibility: PDMS is one of the most flexible chain molecules known, both in the dynamic sense and in the equilibrium sense. Dynamic flexibility is to do with a molecule's ability to change spatial arrangements by rotations around its skeletal bonds. The higher the dynamic flexibility, the more it can be cooled before the chains lose their mobility and flexibility and become glassy. Therefore chains with high dynamic flexibility have very low glass transition temperatures, Tg. If a polymer is exposed to a temperature below Tg, it become brittle, therefore, low values of Tg can be highly useful, especially in fluids and elastomers.
The Tg of PDMS, ~ - 125°C, is the lowest recorded value for common polymers. There are two reasons for this dynamic flexibility. One reason is the very long Si-O skeletal bond, and also the fact that the oxygen skeletal atoms are unhindered by the side groups, the oxygen atoms are as small as an atom can be and still have the multi-valency required to continue the chain structure. Additionally, the Si-O-Si bond angle of ~143°C is much greater than the usual tetrahedral bonding of ~110°. This bond angle has a very high deformability. These characteristics also increase the equilibrium flexibility, which is the ability of a chain to be compact when in the form of a random coil. The equilibrium flexibility has an important effect on the melting point Tm. The very low Tm (-40°C) of PDMS is because of this high equilibrium flexibility.
Permeability: PDMS like with other siloxane polymers have much higher permeability to gases than most other elastomeric materials. Thus, for a long time they have been used as gas separation membranes. Soft contact lenses are made from PDMS. The oxygen needed by the eye must be obtained by inward diffusion from the air rather than through blood vessels. The high oxygen permeability makes PDMS ideal for soft contact lenses, but it is too hydrophobic to be adequately moistened by the tears covering the eye.
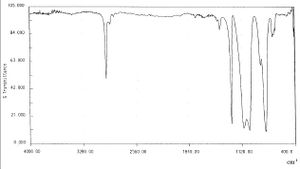
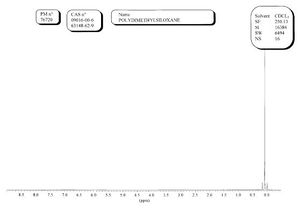
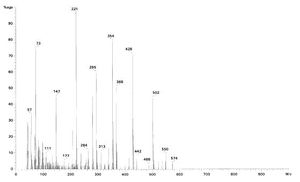
Spectra
 |
 |
 |
|---|
Applications

Medical: There are many medical uses of polydimethylsiloxane. Artificial organs, prosthesis, for example, make use of inertness, stability and flexibility of polydimethylsiloxane. Artificial skin, contact lenses and drug delivery systems also make use of their high permeability.
Non-Medical: High-performance elastomers, membranes, electrical insulators, water repellents, anti-foaming agents, mold-release agents, adhesives, protective coatings, release control agents for agricultural chemicals, encapsulation media, dielectric fluids are all examples. They are based on the properties just mentioned and also their ability to alter surfaces and interfaces (e.g. water repellents, anti-foaming agents and mold-release agents).
References
- Smith, A.L. (1974). Analysis of Silicones. Chemical Analysis Vol.41.
- Clarson S.J., Fitzgerald J.J., Owen M.J. and Smith S.D. Silicones and Silicone-Modified Materials. ACS Symposium Series 729.
- http://cpf.jrc.it/smt/monomers/pm76720.htm
- http://en.wikipedia.org/wiki/Polydimethylsiloxane
