It:Pelargonidin
Flavonoids, Nature's Paintbox
Flavonids are responsible for many bright colours in nature.
In this basic structure only the two end rings are aromatic.
Various different colours can be formed by varying the basic flavonid structure in three main ways, by attaching different groups (mainly OH or OMe) to different points around the rings; by bonding to different sugars (to form glycosides); or by adjusting the acidity of their surroundings.
Structures of Pelargonidin
Pelargonidin is a simple anthocyanidin (a class of flavonid) with the following structure:
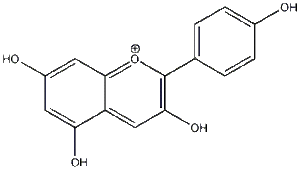
All three rings here are aromatic and the ‘central’ oxygen has a formal charge of +1 (and thus this molecule is sometimes shown with a counter anion such as Cl-).
Colours of Pelargonidin
Pelargonidin itself produces an orange red colour, seen in red geraniums, and ripe raspberries and strawberries to name but a few.
When anthocyanidins bond to sugars they become anthocyanins (a type of glycoside). This is achived by removing a proton from an OH group and bonding to a sugar molecule (such as glucose).


Cyanidin
Cyanidin is also an anthocyanidin and differs from pelargonidin by the addition of an OH to the meta position on the right hand ring and its structure is shown below:

Cyanidin produces a magenta colour, which contributes to the colour of ripe blackcurrants.
An example of how their colour is changed by acidity is shown by cyanidin. Cyanidin is responsible for both the colour of the blue cornflower (in basic conditions) and the red poppy (in acidic conditions).



What is Pelargonidin?
Pelargonidin and Cyanidin are both types of anthocyanidins. These are antioxidants that have been found to help improve blood vessel function in humans and animals.1 Anthocyanidins are found in blue, purple and red fruits and vegetables such as:
- blueberries
- blackberries
- plums
- cranberries
- raspberries
- strawberries.
Anthocyanidins have the general structure:

The different R-groups determine the different types of anthocyanidin. Pelargonidin has a characteristic orange colour and Cyanidin has a characteristic orange-red colour.
Anthocyanidins are found in the cell's cell sap (unlike chlorophyll and carotene that are attatched to cell membranes). The colour of the pigment is altered by the pH of the cell sap. More acidic cell sap gives a red colour. Less acidic cell sap gives a more purple colour.2
Commercial uses of the Pigments
Anthocyanins are used in processed foods (drinks and confectionery) to avoid using synthetic additives. They are used as natural additives because they are brightly coluored and not subject to legal restrictions (as many synthetic additives are). They are also used for their nutraceutical (nutritionally beneficial) properties.
The problem with anthocyanins however, is that they can become instable during processing and storage.3