It:Osmium Tetroxide
Introduction1
Chemical Formula: OsO4 Molar Mass: 254.23 gmol-1 Melting point: 40°C Boiling point: 130°C VERY TOXIC Especially to the eyes. Colourless/Yellow Ozonelike odour
Osmium Tetroxide is an example of the highest oxidation state achieved by transition metal. It has a tetrahedral shape as shown in jmol format below with O=Os=O bond angle of ~109°.
Weapon of terror?
Osmium Tetroxide has been seen as a possible terrorist weapon, this is becuase of its possible use as a catalysist in an explosive device, or due to its high toxicity. The catalytic behaviour is possible due to osmium’s many oxidation states, and the toxicity because of its acidic and corrosive nature in aqueous systems. However it's cost has had it dubbed 'the billionaires weapon'.
Preparation1
Addition of oxygen by burning the metal at 800 °C
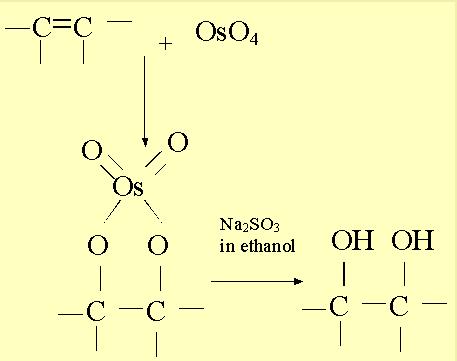
Use in Organic Chemistry
Oxidation of C=C bond1
Osmium forms several oxofluorides, all of which are very sensitive to moisture. Purple cis-OsO2F4 forms at 77 K in an aqueous solution of HF3
OsO4 + 2 KrF2 → cis-OsO2F4 + 2 Kr + O2 OsO4 also reacts with F2 to form yellow OsOF2:
2 OsO4 + 2 F2 → 2 OsO3F2 + O2 OsO4 reacts with one equivalent of [Me4N]F at 298 K and 2 equivalents at 253 K[4]:
OsO4 + [Me4N]F → [Me4N][OsO4F] OsO4 + 2 [Me4N]F → [Me4N]2[cis-OsO4F2]
References
1.http://www.chm.bris.ac.uk/motm/oso4/oso4v.htm
2.http://news.bbc.co.uk/1/hi/uk/3604857.stm
3.Christe et al. Osmium Tetrafluoride Dioxide, cis-OsO2F4. J. Am. Chem. Soc. 1993, 115. 11279-11284