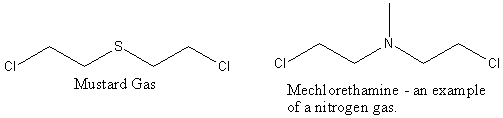
It:Mustard Gas
| Structure of Mustard Gas | ||||
|---|---|---|---|---|
| 3D structure | ||||
| Chemical Names | 1,1-thiobis(2-chloroethane)
bis(2-chloroethyl) [35S]sulfide [35S]sulfur mustard | |||
| Other Names | Mustard Gas | |||
| Molecular formula | C4H8Cl2S | |||
| Molar mass | 157.97 g/mol | |||
| Beilstein Registry Number | 8137686 | |||
| Appearance | Odourless and Colourless gas (when pure) | |||
| Properties | ||||
| Density and phase | 1.27 g/cm3 | |||
| Solubility in water (ph=7) | 0.06% at 20 °C | |||
| Melting point | 14 °C | |||
| Boiling point | 217 °C | |||
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | ||||
Introduction
History
Mustard gas was first used as a chemical weapon in the First World War when it was used to dehabilite the opposing army due to the fact that it could penetrate all known masks and protective clothing used at that time. It has been used as a chemical weapon in various other conflicts since then, most recently in 1988 in Iraq, though it may also have been used throughout the 1990's.
Synthesis
Frederick Guthrie synthesised mustard gas simply by reacting ethene with sulphur dichloride. However, the chemical supplier Bayer AG developed a synthesis during the First World War by which mustard gas can be produced on a mass scale by reacting thiodiglycol with hydrochloric acid.
Chemistry
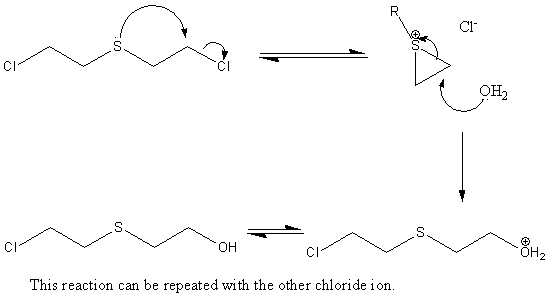
Mustard gas can undergo intramolecular displacement of a chloride ion to form a three-membered cyclic sulphonium ion, (as shown below,) via an SN2 mechanism. Nucleophilic attack on the ion can occur, reacting either with water or proteins in the skin. Mustard gas can react twice in this way as it has two chloride ions that it can lose. Also, having a sulphur atom in the chain causes the SN2 reaction to go much faster than if it was just a straight hydrocarbon chain.
Physicological Effects
Mustard gas is a blister agent that causes pain and irritation to the skin and eyes, however the symptons of exposure only become apparent between two and twenty four hours after the attack. It is known as a blister agent because it causes water blisters on the body of a person who has been exposed to it. This is because despite the fact that mustard gas isn't very soluble in water it is very soluble in fat which means it can be absorbed quickly by the skin. Mustards attack the corneas and lead to blindness, if it is inhaled, it will cause the lining of the lungs to blister which can cause chronic impairment or death. It is possible to treat exposure to mustard gas by detoxification with either sulphur amines, magnesium monoperoxyphthalate or peroxy acids. Exposure to mustard gas can result in permanant disability, cancers or, if more than fifty percent of the body is exposed to mustard, death.
Nitrogen Mustards
Disposal
Mustard gas used to be disposed of by being dumped into the sea which has caused severe problems because the mustard polymerises on contact with the water. This has lead to continued cases of mustard exposure because the polymerised compound can be brought to the surface of the sea by fishing nets. In particular this occurs in Sweden where mustard gas was dumped into the water after the Second World War. Burying mustard gas also creates problems and the gas has been found to still be in the soil ten years after burying. This occurs especially in areas where there is little water in the soil. The mustard gas reacts with the water to form an intermediate sulphonium ion. This sulphonium ion then reacts further with the water to form a stable sulphonium ion that forms a protective layer around the mustard preventing the rest of it from reacting.
References
http://physicsweb.org/articles/world/12/11/10/1/pw-12-11-10fig1
http://www.chm.bris.ac.uk/motm/mustard/mustard2.htm
http://en.wikipedia.org/wiki/Mustard_gas
http://www.opcw.org/resp/html/mustard.html
Jonathan Claydon, Nick Greeves et al, Organic Chemistry, Oxford University Press, 2001, p1258.