It:Morphine
Introduction to Morphine
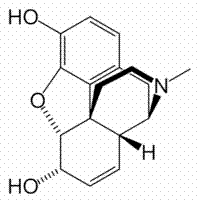
Structure of Morphine
|
|
|
Properties of Morphine
| Chemical Data | |
| IUPAC Name | 7,8-didehydro-
4,5-epoxy-17-methylmorphinan-3,6-diol |
| Chemical formula | C17H19NO3 |
| Molar Mass | 285.4 g/mol |
| Pharmakenetic Data | |
| Bioavailability | ... ~30% |
| Protein binding | ... 30–40% |
| Metabolism | ... Hepatic 90% |
| Therapeutic Considerations | |
| Pregnancy cat. | C(AU) C(US) |
| Legal status | S8(AU) Schedule I(CA) Class A(UK) Schedule II(US) Prescription only |
| Dependence Liability | Extremely High |
| Routes | smoked/inhaled, insufflated, Oral, SC, IM, IV |
Uses
Morphine is used legally:
- analgesic in hospital settings for
- Pain after surgery
- Pain associated with trauma
- In the relief of severe chronic pain
- Cancer pain
- Pain from kidney stones
- Back pain
- In epidural anesthesia
- As an antitussive for severe cough
- As an antidiarrheal in chronic conditions (e.g., for diarrhea associated with AIDS)
- To relieve breathlessness in patients in respiratory failure
Pharmacology of Morphine
Morphine is an opiod receptor agaonist whose main function is to bind and activate the µ-opioid receptors in the central nervous system.The activation of these receptors is what leads to euphoria, sedation, analgesia, physical dependence and respiratory depression. Morphine is also a κ-opioid receptor agonist, which is associated with spinal analgesia and miosis. These effects of Morphine can only be countered with naloxone or naltrexone.
|
|
|||||||
| Naloxone | Naltrexone | ||||||
Both naloxone and naltrexone are competitive antagonists at the μ- and κ-opioid receptors, and to a lesser extent at δ-opioid receptors. The blockade of these receptors is what prevents the opiods such as morphine, from combining with these receptor sites thus preventing the side effects usually caused by morphine.
Derivatives of Morphine
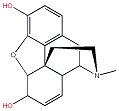
Morphine can be used to synthesize a variety of different opiates. Some of the most prominent examples include:
|
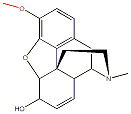
|
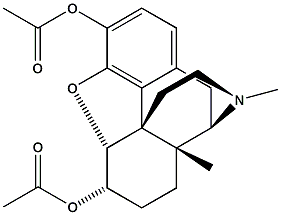
| ||||||
| Morphine | Codeine | Heroin | ||||||
|
|
| |||||||
When comparing the structures of Morphine with those of Codeine and Heroine, it becomes clear that there are only slight differences. If one of the OH groups from Morphine (marked in red) is replaced by a OMe group it becomes codeine. If these -OH groups are both replaced bu CH3COO group it becomes heroin.
Codeine is used as an analgesic, in a similar way to morphine. It is not as effective as morphine, however the addiction potential is much lower than that of morphine.
Heroin acts as a very strong analgesic with an extremely strong potential for addiciotn. For obvoius reasons the synthesis of heroin will be omited.
Contradications
- Acute Respiratory Depression
- Acute Alcoholism
- Acute pancreatitis (this may be a result of morphine use as well)
- Renal failure (due to accumulation of the metabolite morphine-6-glucuronide)
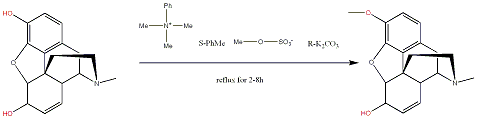
Synthesis of Codeine from Morphine
References and External Links
- Morphine Apparently in Your Head -- Wired Magazine article about endogenous production of morphine
- Morphine, Molecule of the Month
- Opioid Analgesics
- Schmitz J, Stotts A, Rhoades H, Grabowski J. "Naltrexone and relapse prevention treatment for cocaine-dependent patients.". Addict Behav 26 (2): 167-80. PMID 11316375.
- Chicago Recovery Alliance's naloxone distrobution project
- Synthesis of codeine from morphine. Ai, Lin; Chen, Xiaoyuan; Zhang, Le. Faming Zhuanli Shenqing Gongkai Shuomingshu (2005)