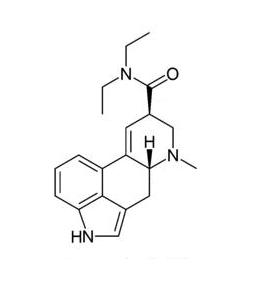
It:Lysergic Acid Diethylamide
Introduction
First synthesised in 1938 Lysergic Acid Diethylamide is a pyschoactive compound more commonly known as the abbreviation LSD. It is a non-toxic, non-addictive drug that mimics serotonin in the brain.
History
LSD was first synthesised in 1938 by Dr. Alfred Hoffman, a Chemist in the pharmacological department in Sandoz Laboratories, Basel, Switzerland. It was initially developed while he was studying derivatives of lysergic acid, systematically reacting the carboxylic acid group with various reagents, to produce the corresponding amides, anhydrides, esters, etc. The reaction of diethylamine with this group produced what was then named LSD-25, however no real benefits of the compound were found and its study was discontinued.
Interest in the drug resumed 5 years later when Dr Hoffman synthesised LSD-25 again so that a sample could be given to the pharmocological department for further testing, when it was thought to be a potential treatment for schizophrenia. Although initial observations on the benefits were highly optimistic, empirical data developed subsequently proved much less promising.
Although LSD is relatively non-toxic and non-addictive, various governments around the world outlawed it after a number of fatal accidents were reported. Such accidents involved, for example, people under the influence of LSD jumping to their deaths off buildings believing they could fly. Research in the 1960's and 70's showed that there was also a considerable psychological risk with the drug and that high doses, especially in inappropriate settings, often caused panic reactions. Scientific study finally ceased around mid 1970's as research funding declined, due to political reasons.
How does LSD work?
Although the mechanism by which LSD causes such profound affects on the human perception still hasn't been established LSD has a very similar molecular structure to a hormone secreted in the brain called Serotonin which is why the body mistakes LSD for serotonin. LSD stimulates centers of the sympathetic nervous system in the midbrain by binding with the 5-HT receptors in the brain. Due to its higher affinity for these receptors serotonin is hindered from sending neural messages in the presence of LSD.
The common functional group found in Serotonin and LSD is the Indole ring (shown in blue below) that is characteristic of the tryptamine molecules and is also found in many other psychodelic compounds such as Psilocybin and Ergoline, which are alkaloids that occur naturally in fungi and plants.
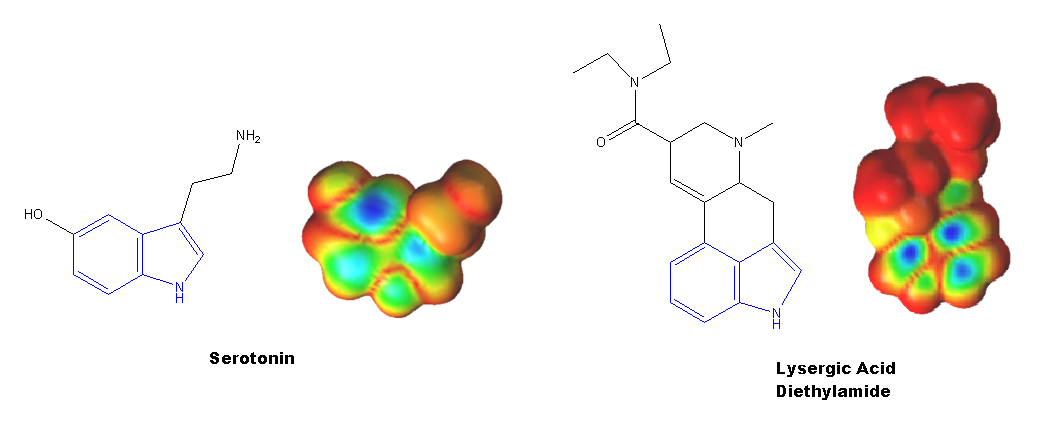
The indole ring has a great influence on the electron density and dipole moment of both molecules and is likely to be responsible for the binding ability of both molecules to the 5-HT receptors.
Synthesis of LSD
LSD is commonly synthesised from lysergic acid, which is made from ergotamine, a substance derived from the ergot fungus on rye, or from ergine (lysergic acid amide), a chemical found in morning glory and hawaiian baby woodrose seeds. The following process is for the LSD synthesis from lysergic acid:
This process produces ~58% of the (+)-D-LSD which is pyschoactive. The Iso-LSD formed is suprisingly non-psychoactive and in the presence of base rapidly interconverts between the isomers. It can also be isolated by chromatography and isomerised to LSD.
References
www.wikipedia.org
www.chemsoc.org/exemplarchem/entries/2004/bristol_rosling/My%20Webs/LSD.htm
www.chm.bris.ac.uk/motm/lsd/lsd1_text.htm
www.coolnurse.com/lsd.htm
Ergot alkaloids. XIX. The use of N,N'-carbonyldiimidazole in the synthesis of amides of D-lysergic, D-dihydrolysergic (I) and l-methyl-D-dihydrolysergic (I) acids. Cerny, A.; Semonsky, M. Vyzkumny Ustav Farm. Biochem., Prague, Collection of Czechoslovak Chemical Communications (1962), 27 1585-92 (SciFinder Scholar)