It:Luminol
Luminol


Background
Luminol exhibits interesting chemistry because it is one of few substances that undergoes reactions which produce light. When in the presence of an oxidising agent, metal catalyst and peroxide, luminol will undergo a reaction which forms a dicarboxylic acid which has electrons in an excited state, these electrons emit light when they fall to their ground state.
The three light emitting processes in chemistry are fluorescence, phosphoresence and chemiluminescence.
Fluorescence is the most common process, and simply involves the absorption and (almost) instantaineous emission of a different wavelength, usually absorbing ultra-violet light and emitting visible wavelengths. The ultraviolet light promotes and electron into a singlet energy state, and charges it with vibrational energy, the electron then undergoes relaxation, during which it loses its vibrational energy, but remains in the singlet state. The electron then emits a photon and falls to the ground state.
Phosphorescence is a less common process because it has to undergo a 'forbidden' electron transition. In phosphorescence light is absorbed, relaxation occurs, then the electron falls to a triplet energy state, which means it has a higher spin multiplicity than the previous state, but lower energy. The electron can remain effectively trapped here for hours because the emission step is spin forbidden, so it occurs slowly. This is the cause of 'glow in the dark' materials.
Chemiluminescence is similar to both of the above, however the energy used to promote an electron to the singlet state comes from a chemical reaction, so no external light source is required. The fall to the ground state can occur either through a fluorescent or phosphorescent path. In the particular case of luminol reactions the process follows a fluorecence like path to the ground state.
Note on Spin: The singlet and triplet state refers to the spin multiplicity of the system. The spin multiplicity is 2S+1, where S is the total spin quantum number of the system. This is calculated from the sum of the spins of the electrons (+/- 1/2). The triplet state has a higher multiplicity than the singlet state.
Data
| Systematic Name | 5-Amino-2,3-dihydro-1,4-phthalazinedione |
|---|---|
| Molecular Formula | C8H7N3O2 |
| Molecular Mass | 177.16 |
| Boiling Point | 319-320oC |
| Physical Form | White Crystalline Solid |
| Hazard Information | Irritant |
| SMILES | NC1=C(C(NNC2=O)=O)C2=CC=C1 |
| CAS Number | [521-31-3] |
Reactions
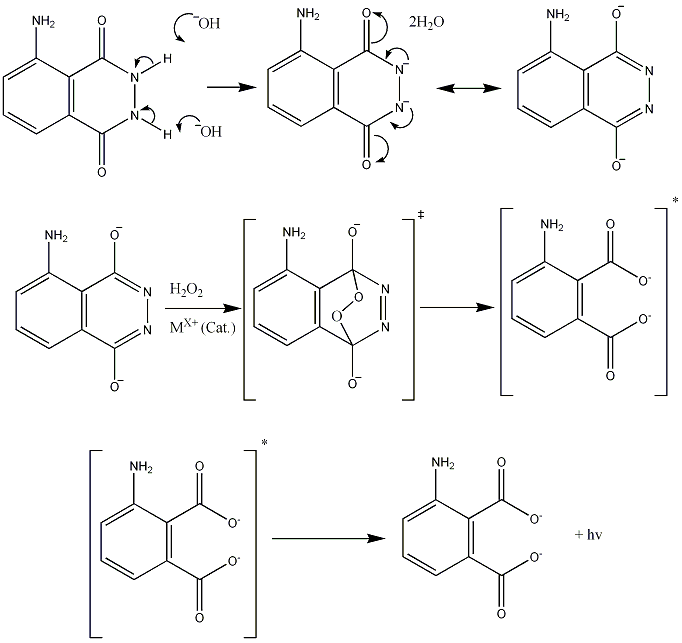
This is the reaction scheme which luminol undergoes. The exact method through which the metal centre catalyses the reaction is still subject to debate, but it is thougth to interact with the peroxide while the bridge forms and the nitrogen gas is eliminated. The star is used to denote a singlet state (i.e unpaired electrons), which then falls to the ground state, emitting light.
Analysis of the light has shown the peak wavelength emitted in the reaction is 460nm, this can be used to calculate the energy transition between the singlet and ground state:
E=hc/λ
=> E=4.321*10^-19 Joules
1eV=1.602*10^-19J
E=2.697eV
For comparison, the 1st Ionisation energy of Hydrogen is 13.6eV
Synthesis
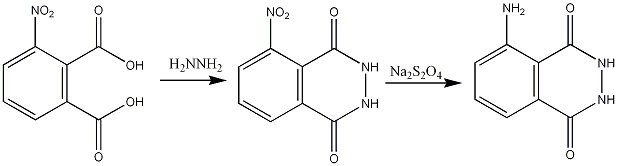
The first step of the synthesis; condensation of 3-aminophthalic acid (commercially available) and hydrazine, must be carried out at high temperature, and requires high boiling point solvents. The reduction of the nitro group with sodium dithionate requires a protic solvent.
Due to the severity of the conditions in the first step it is often impractical to carry out in the lab, however luminol can be bought directly from suppliers[1]
Uses
Luminol is most commonly used in forensic testing to find traces of blood on crime scenes; the haemoglobin in the blood contains enough iron to catalyse the reaction. The solutions used for this kind of investigative work are commonly made up of sodium hydroxide, luminol and hydrogen peroxide in aqueous solution. These solutions are unstable, as the luminol will decompose, so they can only be stored for short lengths of time, in cold dark conditions. Solid luminol is stable, however, and can be stored for prolonged amounts of time.
History
Using luminol on blood samples was first developed by W. Specht in 1937, however it wasn't untill M. Grodsky, in 1951, who developed a solution of sodium bicarbonate and sodium perborate in water that it became useful in an investigative manner. This solution emits light slowly, so it must be used in conjuction with a photography plate to be useful. You could be forgiven for thinking this occurs via the phosphorescent method above, hence the slow nature of the reaction, however it is due to the nature of the solution; sodium perborate (NaBO3) in water will slowly liberate hydrogen peroxide, but not in significant concentrations. In 1966 K. Weber developed the solution above which produces easily visible results and speeds up the anaylsis rapidly.
References
[1] http://www.sigmaaldrich.com/catalog/search/ProductDetail/ALDRICH/123072