It:Fullerene
Fullerene: A brief history
Fullerene is a carbon allotrope that was recently discovered in 1985 by Buckminster Fuller of whom, the molecule is named after. Fuller was not a chemist but an architect and was renowned for the geodesic dome. The interesting shapes of fullerene are in the form of spheres, tubes and ellipsoids that give it a variety of uses. Buckyballs = spherical in the form of C60 and are analogous to the football picture. Buckytubes = cylindrical
Buckminster fullerene is the simplest fullerene with the the formula C60. The odd structure consists of hexagons and pentagons. This fullerene is the most abundant and can be found in soot (ie the carbon residue after combustion).
References: http://en.wikipedia.org/wiki/Fullerene
Structure:
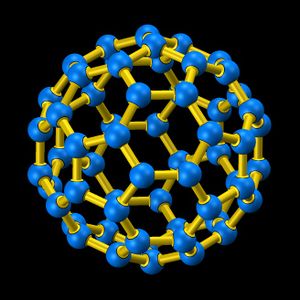
The following blue and yellow image was taken from http://www.chem.sunysb.edu/msl/fullerene.html.

The icosahedral cage picture was taken from the fullerene wiki page.
The diagram above shows fullerene C60 in the centre reacting with the neighbouring reactants. The following structures were taken from Conquest by saving it as a PDB file and then copying and pasting the codes into the Wiki page.
Reactions
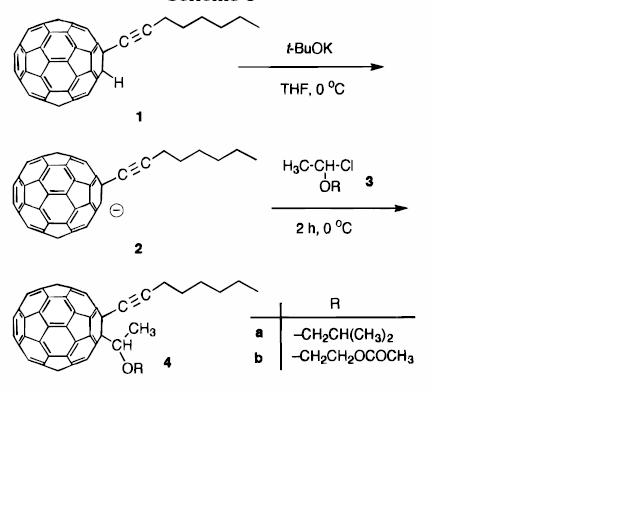
The above reactions show the synthesis of disubstituted 1,2-dihydro[60]fullerenes. The use of this reaction is to improve the solubility and processability of C60 fullerene. First of all, the fullerene is converted into a stable carbanion of C60 2-(1-octynyl)-1,2-dihydro[60]fulleren-1-ide ion. Then the carbanion will react with the carbon electrophiles given from vinyl ethers.
Reaction of fullerene with Grignard reagents
The specific reaction that I will be dealing with is C60 fullerene reacting with a phenyl grignard reagent in the presence of Cu(I) salt to give an addition product C60Ph5H. The yield for this reaction is very high (98%). From the diagram below, you can see that adduct has five R groups attached to a protonated cyclopentadienyl anion, implying there is nucleophilic attack of just one face.
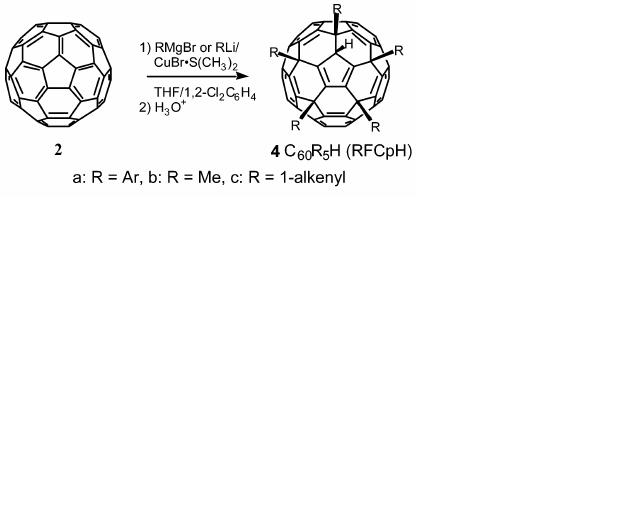
C70 can also react with the grignard reagent in the same way but instead of a penta-adduct product, we have a tri-adduct product C70R3H. Once again the yield is exceptionally high (90-99%). The product formed leads to the discussion of carbon clusters.

The 2 above reactions have been referenced from http://www.iupac.org/publications/pac/2003/pdf/7504x0427.pdf
Bucky Ferrocene and Rh
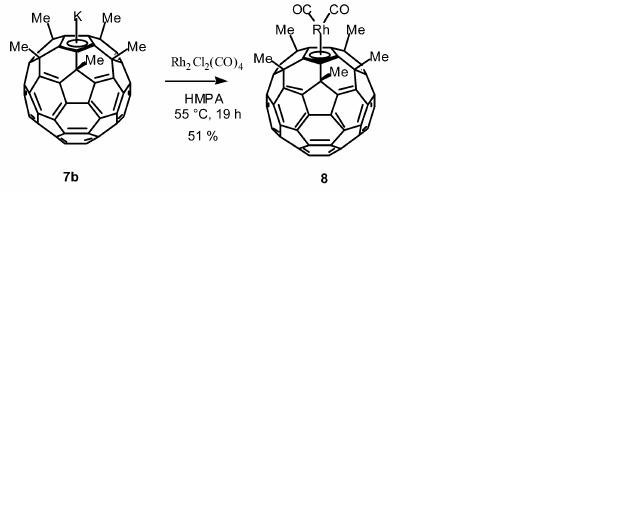
The first transition metal complex with Rh and bucky ferrocene made together was Rh(η5-MeFCp)(CO)2. This is made by the transmetalation reaction of KMeFCp and Rh2Cl2(CO)4 in hexamethylphosphoramide. The final transtition metal complex product is relatively stable against air and mild acid/base conditions. The transition metal Rh now adds an 'extended electronic array' and therefore improving the superconductivity of fullerene. The MeFCp complex can be oxidised and reduced without losing the Rh atom and this is a reason why this type of compound is so useful as a catalyst.
Uses of fullerenes:
With the entrance of fullerenes into chemistry, modern fullerene chemistry is getting more and more promising in terms of modern uses. The Buckminster fullerene unit can be used as 'magnetic nanoparticles' that pave the way for the magnetic storage of high density media. Also, the fullerene structure can be placed into ferrous materials such as iron and cobalt to give thin films with high magnetic properties - this may extensively be used in computers.
Another odd structure is the ferrocene one and fullerene can be made to resemble the ferrocene structure with a Rh centre. This leads to the super conducting properties of fullerene.
Of late, carbon nanotubes are 'new and fashionable' materials with a hollow cylinder structure as shown below. The structure resemblance is that of graphite and as you can see, hexagonal C rings form the wall structure. We can have two types of C nanotubes:
1, single-walled nanotubes
2, multi-walled nanotubes
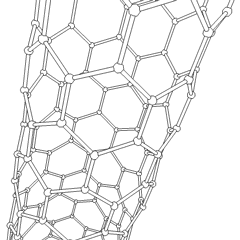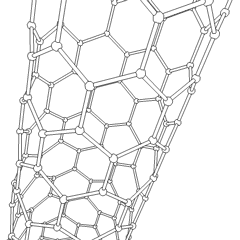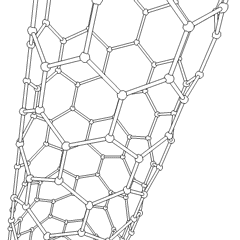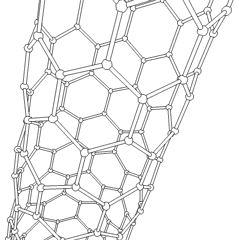
The interest in C nanotubes is that they are both rigid and flexible at the same time. For example, in comparison to the stress strength of steel, the nanotube is a hundred times stronger than steel. The main use of the nanotubes is for its superconductivity properties. The conductivity refers to both thermal and electrical. Some major uses of the C nanotube are as a field emitter and as a semiconducting molecule.
The rapid research developments in the nanotube field has now asked questions about whether silicon components for computers will still be around in a few decades time, or if the nanotube materials will replace this 'old' material.
