It:Epibatidine
| Structure of Epibatidine | ||||
|---|---|---|---|---|
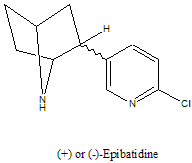
| ||||
| 3D structure | ||||
| General Properties | ||||
| Chemical Name | (+/-)-epibatidine | |||
| Auto name/|Systematic name | 2-(6-chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]heptane | |||
| Molecular formula | C11H13ClN2 | |||
| SMILES | ClC1=CC=C(C2C(N3)CCC3C2)C=N1 | |||
| Molar mass | 208.69 g/mol | |||
| Beilstein Registry number | 6139872 | |||
| Type of Substance | heterocyclic | |||
| CAS number | 140111-52-0; 148152-66-3 | |||
| Lawson Number | 28122 | |||
| Appearance | White Solid | |||
| Chemical Class | Alkaloid | |||
| Natural isomer | (+)-epibatidine | |||
| Solubility | soluble in ethanol (20mg/mL) | |||
| Physical Properties | ||||
| Melting point | 59-66°C | |||
| Boiling point | ||||
| Optical Rotation of (-)-epibatidine | [α]D -5.2° (in chloroform) | |||
| Optical Rotation of (+)-epibatidine | [α]D +5.4° (in chloroform) | |||
| Hazards | ||||
| Main hazards | ||||
| Analytical data | ||||
| Spectral data | NMR, UV, IR, MS | |||
| Related compounds | ||||
| ABT-594 | ||||
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references] | ||||
Epibatidine: An Introduction
Epibatidine was first extracted from the skin of a poisonous Ecuadorian frog, namely Epipedobates Tricolor, in 1976 by John Daly. At the time it was simply called alkaloid 208/210 (its molecular mass determined via mass spectrometry). Originally he could not obtain the definitive structure of the sample he had collected (which was less than 1mg) because the analytical tools at the time did not allow him to work with such a minute quantity. He also could not extract any more from the rare frog because this particular frog species had been declared endangerd, so the sample was frozen and stored for the best part of a decade before its structure was fully declared after the development of NMR spectroscopy in 1986.1
An attempt was made to breed these unique frogs in captivity to provide a source of epibatidine. A remarkable result was deduced. The frogs bred in captivity produced no epibatidine. The researchers logically concluded that the frogs obtain this naturally scarce compound from their diets and environment.1
Daly obtained evidence that this compound was a potent analgesic. In the hot plate assay and Straub-tail assays respectively it was found to be 200-500 times more potent than morphine.5 Moreover, epibatidine was found to have negligible (1/8000) affinity for opiate receptors (it is not an opioid like morphine). Daly showed that naloxone (an opioid antagonist) did not block the action of epibatidine. The significance of this discovery is that it provides strong evidence that epibatidine is not addictive, unlike morphine.1
It was observed that epibatidine has a similar structure to nicotine. Soon after it was found that epibatidine binds to nicotinic acetylcholine receptors.1 This provided a solution to its analgesic powers. However, the potential of epibatidine as an analgesic was ended when it was found that it binds very strongly to the neuromuscular receptor in which acetyl choline regulates muscle contraction and relaxation. As a result epibatidine causes paralysis and can be fatal if the muscles in conjunction with the respiratory system become depressed.1
ABT-594 – An Analogue of epibatidine: a possible future analgesic?
Epibatidine was determined useless as an analgesic due to its toxicity. Nonetheless, researchers were excited by the possibility of developing an analgesic through epibatidine-like derivatives that did not render toxicity.
Among the many tested, ABT-594 is the most famous example and was found to be 50 times more potent that morphine as well as not possessing its addictive nature. More importantly it also demonstrated no cases of paralysis or depression of muscle action during clinical trials. This drug has undergone Phase I trials with success and has recently completed its latest trial set, Phase II, although the results of the latter trial are yet to be published.1
Synthesis1+6
There are two optical isomers of epibatidine. The (+)-epibatidine isomer is the naturally occurring isomer that is found on the skin of the Epipedobates Tricolor frog. However, the (-)-epibatidine isomer also possesses the analgesic properties so sought after, so a racemic mixture of the two isomers would be just as effective as a painkiller.
There have been many different synthetic routes to attempt to produce epibatidine in the past, as it has been such a sought after compound for research. A variety of synthetic strategies have also been employed.
The Corey Synthesis
This synthesis was developed in 1993, just one year after Daly had released the structure of epibatidine. It is stereospecific, in that the endo-form is not produced. The enantiomers of epibatidine that are produced as a racemic mixture from this synthesis can be separated by liquid chromatography, due to their different eluting times, if desired.

The first step involves converting the starting material to the (Z)-stereoisomer of the α,β-unsaturated ester with the aid of the Still-Gennari reagent. This mechanism is stereospecific because the transition state corresponding to that of the (Z)-stereoisomer is less hindered and therefore the product formed via this route is more thermodynamically stable. The second step involves a simple thermal Diels-Alder reaction, performed at 190°C.

In Step 3 saponification of the ester forms the corresponding carboxylic acid, which is in turn transformed into an acyl azide (Step 4). The removal of the TMS protecting group is then performed via a carbamate cleavage to give the corresponding primary amine. This amine then undergoes an acylation reaction with tetrafluroacetic acid, producing Compound 7.

The production of compound 8 requires the key stereospecific halogenation, in the presence of bromine and a source of the bromide ion. Stereospecificity is due to the kinetically favoured attack of the bromide ion cis to the NHCOCF3 group. An excess source of bromide ion (10 mole equivalents) is fundamental to the stereospecific nature for this reaction, as its presence directly stabilises the cis-bromium ion. The formation of the bicyclic ring can then proceed under basic conditions via nucleophilic attack (Step 7). Debromination and deacetylation in Steps 8 and 9 respectively lead to the desired product.
Each step in this synthesis is performed with a fairly high yield culminating in an overall yield of 40%.
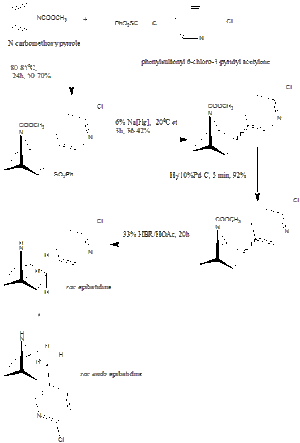
Huang and Shen
This synthetic route (published in 1993 as well) is perhaps conceptually simpler than the Corey Synthesis, although it produces a lower yield, 25% of the epibatidine. Another problem with this synthesis is that it also produces the endo-isomer of epibatidine (with a yield of 28%), although silica gel chromatography can used to separate this isomer from the desired exo-isomer.
The first step involves a Diels-Alder reaction involving the two starting materials: N-carbomethoxypyrrole and phenylsulfonyl 6-chloro-3-pyridyl acetylene. The two unwanted double bonds were then reduced by first sodium amalgam and then a Platinum catalyst and hydrogen. The final step involves the removal of the N-carbomethoxy protecting group to reveal the desired product.
References
1. Jenny Pan, 2004, http://www.chemsoc.org/ExemplarChem/entries/2004/icl_Pan/page2.html
2. The Science Center, 2004, http://www.science-education.org/classroom_activities/chlorine_compound/epibatidine.html
3. Matthew J. Dowd, Department of Medicinal Chemistry of Virginia Commonwealth University, http://www.phc.vcu.edu/Feature/oldfeature/epi/
4 Chris A Broka, Tetrahedron Lett., 1993, Volume 34, Issue 20, 3251-3254, DOI:10.1016/S0040-4039(00)73674-4
5. Dao Fei Huang, T. Y. Shen, Tetrahedron Lett., 1993, Volume 34, Issue 28, 4477-4480, DOI:10.1016/0040-4039(93)88063-O
6. E. J. Corey, Teck-Peng Loh, Sidduri AchyuthaRao, Donnette C Daley, Sepehr Sarshar, J Org. Chem, 1993, 58, 5600-5602, DOI:10.1021/jo00073a013
