It:Cyclamate
Introduction and Historical Background
Cyclamate, also known as (E952) is an artificial sweetener that was discovered in 1937 at the University of Illinois. It was discovered by a graduate student, Michael Sveda who discovered it completely unknowingly. He was working on the synthesis of anti-pyretic drugs, while smoking a cigarette. He put his cigarette down on the lab bench, and to his surprise when he put it back in his mouth, he has discovered the sweetener cyclamate. The first patent for cyclamate was bought by DuPont, and then later sold to Abbott industries who conducted further research and tests. in 1950, they apllied for a New Drug Application for cyclamate. Cyclamate is the sodium or calcium salt of cyclamic acid(cyclohexanesulfamic acid). They have the same uses.
Uses
Initially, Abbott industries had intended to use cyclamate for masking the bitter taste of certain drugs such as antibiotics. However, it was later developed in tablet form, aimed specifically at diabetics as a sweetener. Cyclamate was in use until 1969 as an artificial sweetener, until their use was banned by the U.S. Food and Drug Administration after reports that large quantities of cyclamates could cause cancer in some animals. It is also available in packet or liquid form. As cyclamate is stable in heat, it can be used in cooking and baking. Commercially, it is available as Sucaryl™.
Synthesis
Cyclamate can be prepared in a different ways. Cyclohexanamine can be reacted with chlorosulfonic acid give to N-Cyclohexylsulfamic acid, which when reacted with for example sodium hydroxide, yielding the sodium cyclamate. Instead of chlorosulfonic acid, sodium chlorosulfonate could be used, which leaves out the final step of adding the sodium hydroxide. However, this involves a longer experimental procedure, and so is not a preferred method of synthesis.
Alternatively, a process known as the nitridation of sodium dithionite. Using sodium dithionite and certain nitro compunds, sulfamic acids can be yielded. The mechanism occurs in two steps. This reaction is done in the presence of excess hydroxyl ion is to prevent the solution from becoming acid, which would leads to decomposition. Tertiary sodium phosphate can be used for this purpose.
There are many other synthethic routes that could be taken - see the reference: Journal of Organic Chemistry, 9, 89-101; 1944 for further details.
Intersting facts
- Cyclamate is 30–50 times sweeter than sugar.
- It is often used with artificial sweeteners such as Saccharin in a mixture of 10 parts cyclamate to 1 part saccharin. This mixture is used as it masks the the off-tastes of both sweeteners.
- Between 1963 and 1970 national consumption of cyclamates--in soft drinks, canned fruits, candy, salad dressings and other foods--soared to 21 million pounds annually from 5 million.
- It is also cheaper than most sweeteners.
- Many companies advertised the banning of Cyclamate on their products e.g. Dr Pepper Soft Drinks
Risks and Controversy
In 1969, cyclamate was banned from use by the USA Food and Drug administration for its possible risk of bladder cancer in humans. This was found from tests conducted on animals by Abbott Laboratories. They found that 8 out of 240 rats fed a mixture of saccharin and cyclamates (1 to 10 ratio) at levels of humans drinking 350 cans of diet soft drinks a day developed bladder tumours. This led to the FDA banning their use for human consumption. More than 50 countries continued using them as a sugar substitute. However, recent animal studies have failed to show that it is a carcinogen (cancer causing agent) or a co-carcinogen (enhances the effect of a cancer causing agent). But before it is readministered for use many other issues must be resolved.
Cyclamate is not used in commercial products, and is only available as a "table top" sweetener. Cyclamate is also not recommended for use during pregnancy, unless under the advice of a medical practioner.
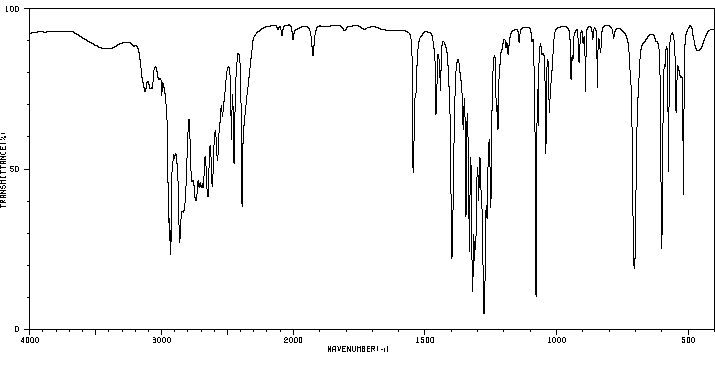
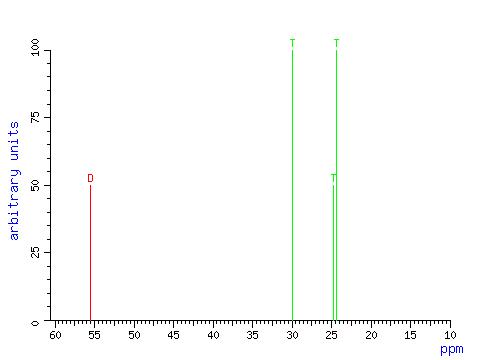
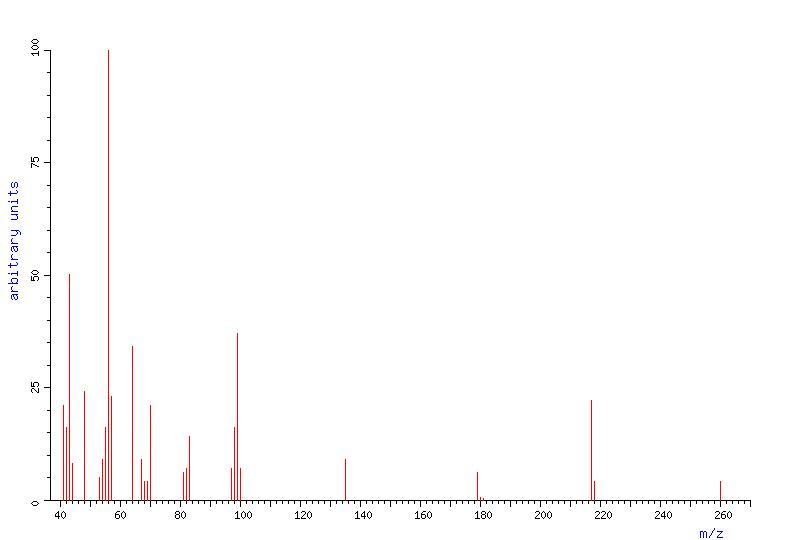
Spectra
The following are the spectra obtained of cyclamate, useful for chemical investigation:
References
- Sucryl™ Image taken from http://www.calgaryhealthregion.ca/clin/women/images
- Dr Pepper image taken from www.personalpages.bellsouth.net/s/k/skytrix3/n042.jpg
- Journal of Organic Chemistry, Audrieth and Sveda, 9, 89-101; 1944
- http://en.wikipedia.org/wiki/Cyclamate
- http://www.caloriecontrol.org/cyclam2.html
- http://www.cyclamate.com/
- http://www.elmhurst.edu/~chm/vchembook/549cyclamate.html
- http://www.encyclopedia.com/doc/1E1-cyclamat.html
- SciFinder Scholar, 2006