It:Cinnamaldehyde
Introduction
| Cinnamaldehyde | |
|---|---|
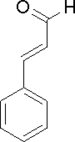
| |
| General | |
| Systematic name | 3-phenyl-2-propenal |
| Other names | trans-cinnamaldehyde,
cinnamic aldehyde, trans-cinnamic aldehyde |
| Physical Appearance | Yellow oily liquid |
| Molecular Formula | C6H5CH:CHCHO |
| Molar Mass | 132.16 |
| SMILES | O=C([H])/C=C/C1=CC=CC=C1 |
| CAS Number | 104-55-2 |
| Pysical Properties | |
| Melting Point | |
| Boiling Point | 248 °C(lit.) |
| Flash Point | 160 F |
| Density | 1.05 g/mL at 25 °C(lit.) |
| Refractive Index (n20/D) | 1.621(lit.) |
| Vapour Density | 4.5 (air = 1) |
| Vapour Pressure | 0.02 mm Hg at 20 C |
| Solubility | Slight in water |
| Stability | |
| Stability | Stable, but combustible. Incompatible
with strong bases and oxidising agents. |
| Spectral Data | |
| Spectra | UV/Vis, Mass, IR |
| Hazards and Safety | |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38-R43 |
| Safety Phrases | R26-R36/37 |
Cinnamaldehyde is an unsaturated aldehyde found in the bark of the Cinnamomum zeylanicum tree. It is a component of the spice Cinnamon, which due to its distinctive flavour and aroma is a common component of among others, foodstuffs, perfumes, and detergents. Cinnamaldehyde can be obtained naturally from the steam distillation of twigs and leaves of Cinnamomum.
Chemical Properties
Cinnamaldehyde is a planar acrylic aldehyde with a benzene ring substituent. Its geometry arises from the presence of the double bond and the aromatic benzene ring. Cis and trans isomers both exist, although the predominant form is the trans isomer, where the terminal carbonyl group is on the opposite side of the benzene ring over the rigid double bond.[1]
Synthesis
Although the majority of cinnamaldehyde is obtained from the steam distillation of twigs and leaves from the Cinnamonium tree, several synthetic methods have been devised. The most common of these are: by condensation of benzaldehyde and acetaldehyde2; condensation of styrene and formyl methylaniline3; and oxidation of the primary alcohol cinnamyl alcohol4
Synthesis by Aldol Condensation of Benzaldehyde and Acetaldehyde This involves reacting benzaldehyde with acetaldehyde in aqueous NaOH with a benzene catalyst, with an optimum yield of 57%5 A reaction scheme for this synthesis follows:
Synthesis by oxidation of cinnamyl alcohol
The alcohol is usually oxidised by an agent such as a chromium(VI) oxide-pyridine complex, which has been shown to be effective at oxidation at room temperature and selective towards the alcohol group rather than the double bond present. This method results in yield of 81% of the product.4
Antimicrobial Properties
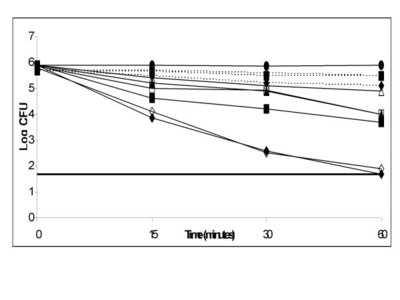
Recent studies have shown that cinnamaldehyde has antimicrobial properties, particularly against the human gastric pathogen Helicobacter pylori, which is a major factor in many gastronomic diseases. It was found that cinnamaldehyde (along with eugenol) was effective in inhibiting all strains of H. pylori, increasing effectiveness at acidic pH. Furthermore, no resistance to this compound was seen even after 10 doses. This is of particular importance as the antibiotic resistance of this bacterium grows: safe and non-antibiotic methods of eradication are required.6
Antifungal Properties
Cinnamaldehyde has also been found to exhibit antifungal properties, and of all the leaf oils of the Cinnamonuium tree, possesses the strongest of these against some strains of fungi (such as Coriolus versicolor and Laetiporus sulphureus).7
Pesticidal Properties
Cinnamaldehyde has been increasingly used as an alternative to conventional pesticides. It has shown particular effectiveness in its uses as an antifungal agent, corn rootworm attractant and a dog and cat repellent. It has become increasingly popular due to its lack of unforeseen adversable effects to humans, non-target organisms and the environment. In addition, its status as a natural extract makes it less expensive to register under the US Environmental Protection Agency due to relaxed regulations regarding natural product based repellents.8
Its use as a bird repellent has been considered, as it contains many of the chemical properties regarded as effective in this area: namely its planar structure; posession of a benxene ring; and its weak basicity at the carbonyl oxygen. However, studies showed that it lost effectiveness after the first day of the trial, suggesting that its potency is not sufficient for sustained use as a bird repellent.9
Notes
1. Chemical Land21-Cinnamaldehyde Information Page
2. Qufu Shifan Daxue Xuebao, Ziran Kexueban, 31(2) 96-98, (2005)
3. GB Patent 504125 (1939 to I. G. Farben)
4. Study of the Chromium(VI) Oxide-Pyridine Complex, Holum J. R., 26, 4814 (1961)
5. Qufu Shifan Daxue Xuebao, Ziran Kexueban, 31(2) 96-98, (2005)
6. Annals of Clinical Microbiology and Antimicrobials, 2005, 4:20
7. WANG Sheng-Yang, CHEN Pin-Fun , CHANG Shang-Tzen: Bioresour. technol., 2005, vol. 96, no7, pp. 813-818
8. EPA Pesticide Factsheet for Cinnamaldehyde
9. http://www3.interscience.wiley.com/cgi-in/fulltext/63003418/PDFSTART
MSDS Resources:

