It:Carmoisine
Introduction
| Carmoisine | ||||
|---|---|---|---|---|
| ||||
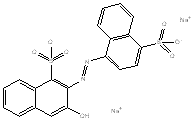
| Rotatable structure of Carmoisine (excluding Na+ cations) | ||||
| General Chemical Data | ||||
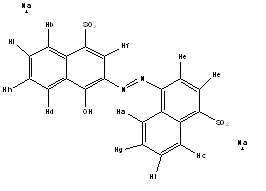
| Systematic name | 2-(4-sulfo-1-Napthylazo) -1-Naphthol-4-sulfonic acid | |||
| Molecular formula | C20H12N2Na2O7S2 | |||
| SMILES | OC2=CC1=CC=CC=C1C(S([O-]) (=O)=O)=C2/N=N/C3=CC=C(S([O-]) (=O)=O)C(C)=C3/C=C.[Na+].[Na+] | |||
| Molecular weight | 502.42gmol-1 | |||
| Appearance | Red powder | |||
| CAS number | [3567-69-9] | |||
| HS code | [3204.12] | |||
| Einecs number | [222-657-4] | |||
| Solubility (in water) | soluble | |||
| Stability | Stable in ordinary conditions2 | |||
| Melting Point (degrees celsius) | >300 | |||
| pH | 6-8 | |||
| Synonyms | CI Food Red 3 | |||
| Density | 0.80gcm-1 | |||
Carmoisine also commonly known as Azorubine and is a red azo dye (a dye derived from an amino compound) that is used in sweets, for example jellies, as well as marzipan, packet cheesecake, packet soup mix and prepackaged sponge puddings or swiss rolls. It has the E number E122. On a more scientific note, it is used in the photometric determination of Mg, Pd, Cu, Sn and Cr.1
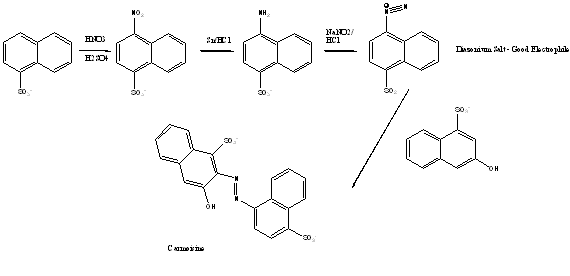
Synthesis of Carmoisine
The first step involves using an alkylated aromatic ring, which is firstly nitrated using concentrated HNO3 and concentrated H2SO4 and then reduced using Sn/HCl to a diazo component. That is to say, a primary aromatic amine. Using acidic conditions (HCl) and NaNO2 an unstable diazonium salt is produced. This is a good electrophile for activated aromatic rings. The process of making this diazonium salt is known as Diazotisation. Then, a coupling component is introduced with which to react this diazonium salt. This forms a the stable azo dye.3
Therefore in the case of Carmoisine, the following would occur:
Structure
As the N=N bond is planar in carmoisine, (as it is in all azo dyes) to geometric isomers are possible, notably cis and trans isomers. The trans isomer is energetically more stable than the cis isomer as clearly there would be a preferance to having the bulky naphthalene groups on opposite sides on the molecule instead of next to each other.
General properties of Azo dyes
The biggest advantage of azo dyes over other types of dyes is that they are cheap and cost-effective. Since only two organic compounds are required to make an azo dye, there is a lot of flexibility in what dyes are made as these two organic compounds can be altered straightforwardly. They are also environmentally sound as their synthesis occurs in water and hence easily disposed of. Lastly, it is easy to scale the relative proportions of each reactant up or down as desired due to the simplicity of the chemistry involved i the dye production.
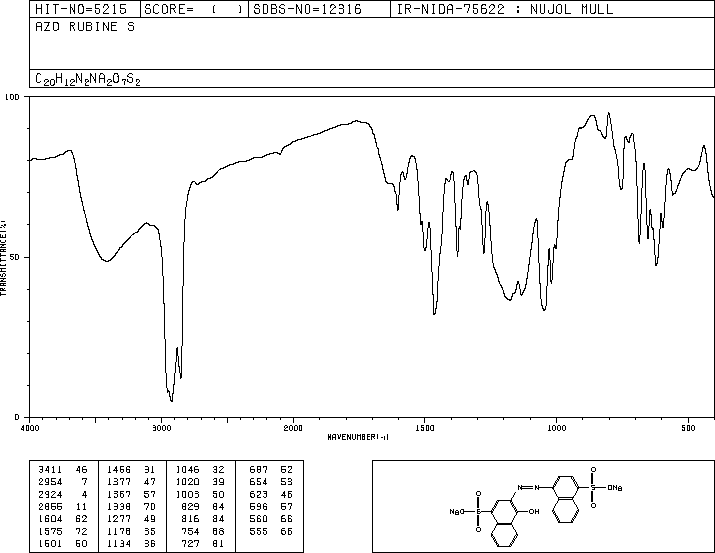
Spectra of Carmoisine
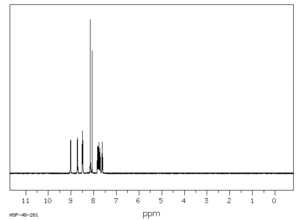
1H nmr spectrum of Carmoisine5
| Proton | Chemical Shift |
| Ha | 9.02 |
| Hb | 8.71 |
| Hc | 8.50 |
| Hd | 8.48 |
| He | 8.15 |
| Hf | 8.07 |
| Hg | 7.82 |
| Hh | 7.78 |
| Hi | 7.20 |
| Hj | 7.63 |
 |
 |
The IR spectrum can be assigned as follows:
| Group | Wavenumber/cm-1 |
| O-H | 3411 |
| C-H | 2954 |
| N=N | 1500-1400 |
| S=O | 1100 |
The aromatic stretches/bends of napthalene are present in the 800-500cm-1 range. This is deep inside the fingerprint region, so-called as it provides a unique 'fingerprint' for each molecule. Thus by analysis of the fingerprint region, the compound in question can be determined by comparing it to a known source from a already existing database.
Adverse Effects
Carmoisine is believed to be linked with hyperactivity in children, with the hyperactive children's support group (HCSG) recommending that it is banned from a child's diet. Also causes reactions in sensitive people with for example an aspirin allergy or asthma. Skin rashes such as urticaria or swelling e.g. odeme are some of these reactions. 4 Carmoisine is prohibited in Japan, Norway, Sweden and the US.
References
1. http://www.ukfoodguide.net/e122.htm
2. http://www.chemicalland21.com
3. 'Organic Chemistry' by Clayden, Greeves, Warren and Wothers page 572