It:Carminic acid
| Carminic Acid | |||
|---|---|---|---|
| |||
| 3D Structure | |||
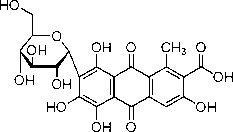
| Chemical name | 7-α-D-Glucopyranosyl-9,10-dihydro- 3,5,6,8-tetrahydroxy-1-methyl-9,10- dioxoanthracenecarboxylic acid | ||
| Other names | Carminic acid Natural Red 4 | ||
| Chemical formula | C22H20O13 | ||
| Colour and state | Bright red, pink to magenta powder | ||
| Molecular mass | 492.38 g/mol | ||
| CAS number | 1260-17-9 | ||
| Melting point | 120 °C (decomp.) | ||
| SMILES | O=C1C4=C(C(C)=C(C(O)=O)C(O)=C4) C(C2=C1C(O)=C(O)[C@@]([C@@H]3[C@H](O) [C@@H](O)[C@H](O)[C@@H](CO)O3)=C2O)=O | ||
| Disclaimer and references | |||
Carminic acid (C22H20O13) is the active ingredient in the food colorant cochineal.
Cochineal was first extracted by drying and crushing the female bodies of the cactus dwelling Dactylopius coccus insect.
The dried cochineal contains 17-24% carminic acid which is then refined to get carmine.
Cochineal was once an extremely valuable commodity and at one point reaching higher value than that of gold. This method is still in practice, but due to the high cost and somewhat unsavoury method of extraction this colorant has now been synthesised (synthesis found below) or been replaced with other synthetic compounds. The first synthesis of the compound was published in 1991 by Pietro Allevi et al.
First Synthesis of Carminic Acid DOI: 10.1039/C39910001319
The compound is soluble in water, mineral acids, ether, alkali
Some spectroscopic data of the compound can be found by clicking links below.
References
Merck Index
http://www.sigmaaldrich.com/catalog/search/ProductDetail/FLUKA/22010
http://www.foodadditivesworld.com/carminic-acid.html
http://chemicalland21.com/lifescience/foco/CARMINIC%20ACID.htm
