It:Caramel
Caramel 2D structure
| Caramel: Information | |
|---|---|
| Systematic name | 4,5-Dimethyl-3-hydroxy-2,5-dihydrofuran-2-one |
| Other names | Caramel Furanone, Sotolon, Sugar Lactone |
| Molecular formula | C6H8O3 |
| SMILES | O=C1OC(C)C(C)=C1O |
| Molar mass | 128.13g/mol |
| Appearance | Orange/brown liquid |
| CAS number | 28664-35-9 |
| FEMA Number | 3634 |
| EG/EC Number | 2491364 |
| Council of Europe No. | 11834 |
| MDL Number | MFCD00059957 |
| Caramel: Properties | |
| Boiling Point | 184°C |
| Melting Point | 26-29°C |
| Refractive Index | n 20/D 1.492 |
| Hazard Information | Harmful by excess inhalation/swallowing |
Caramel is a compound that has been used for a long time in many different foods and drinks due to its sweet taste. It is known under different names such as sugar lactone, caramel furanone (after its chemical name) and sotolon, but it is best known as caramel, after which the food caramel takes its name. It has been designated an E number, E150, as it is used so often in food products as a colouring agent.
3D structure
Uses of Caramel
Caramel has many uses, mostly in food due to its sweet taste but there are less-known uses as a colouring agent:
• Coca cola and other drinks • Caramel sweets (carambar etc.) • Desserts (creme brulee, creme caramel) • Praline • Nougat • Syrup • Tobacco • Cosmetics • Detergents • Dairy Products
Syntheses
The formation of caramel can happen through two different processes, one being the Maillard Reaction and the other being caramelisation. These are both examples of non-enzymatic browning where sugar reacts to form a multitude of products, one of course being caramel.
Maillard Reaction:
- The carbonyl group on a sugar reacts with the amino group of an amino acid in a condensation
- A molecule of water is lost to make N-substituted aldosylamine
- This compound undergoes Amadori Rearrangement to form ketosamines
- From here many products can be formed, although the required one first involves hydrolytic fission producing diacetyls etc
- These diacetyls and other products undergo Strecker Degradation to aldehydes and then condensation to aldols
- These finally produce the heterocyclic compounds with pleasant aromas including Caramel Furanone.
Caramelisation:
- Caramelisation is in general a simpler process.
- In this reaction the sugars lose water molecules from their structure through a process called "1,2 & 2,3-enolisation".
- The product then fragments to make many compounds including caramel.
The sweet caramel can be made through a more familiar "cooking" method by boiling together milk, sugar, butter, oil, syrup, vanilla essence, water, and glucose at about 170 degrees Celcius.
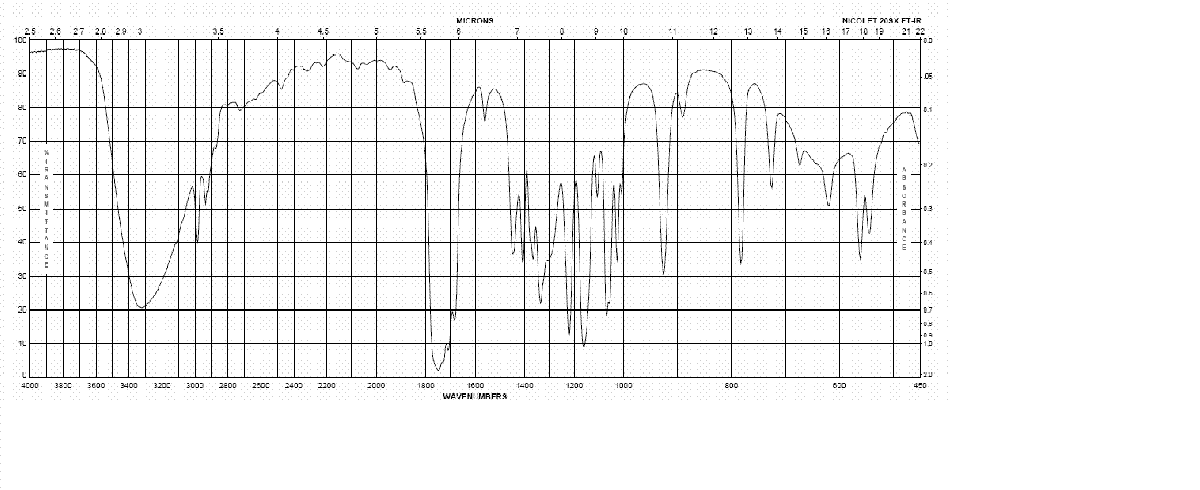
IR spectrum of Caramel
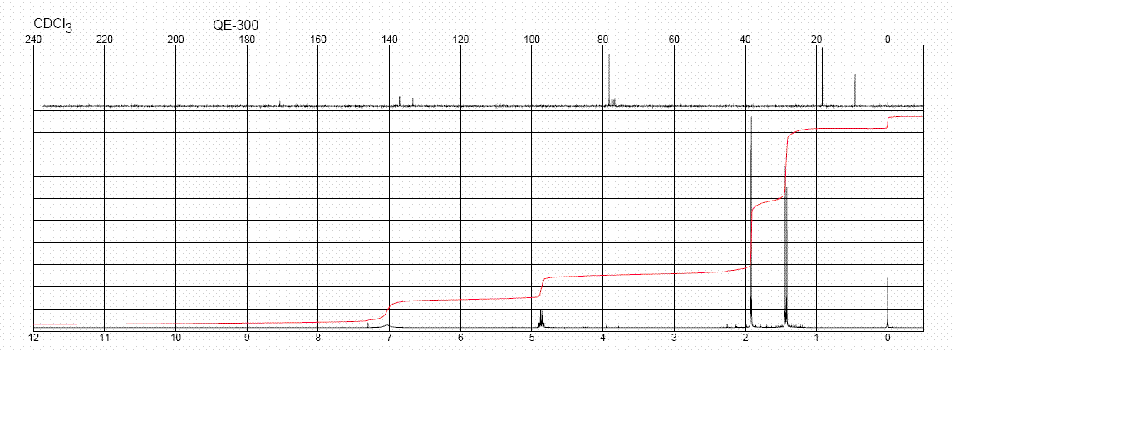
HNMR and CNMR of Caramel
List of References
http://www.sigmaaldrich.com/catalog/search/ProductDetail/ALDRICH/W363405
http://www.pharm-marketing.com/flavor_eng.htm
http://en.wikipedia.org/wiki/Caramel
http://brewery.org/library/Maillard_CS0497.html
(syntheses)