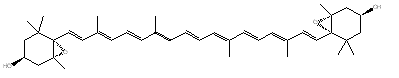
It:Capsanthin
Capsanthin
Introduction
Capsanthin is used as a flavour enhancer in foods and is of the same overall classification as beta-carotene. That is to say, it is a carotenoid, a naturally occuring organic pigment found in plants. The carotenoids are separated into two separate groups, the Xanthophyll's and the carotenes. Capsanthin makes up 30-60% of the carotenoids, and is a Xanthphyll - responsible for the red colour in peppers and paprika.1 Lastly, Capsanthin may also be used to enhance the colour of egg yolks by adding it to poultry feed.
Capsanthin rotatable structure
| Template:General chemical data | It:Capsanthin | |
|---|---|
| |
| Systematic name | (3R,3'S,S'R)-3,3'-dihydroxy-beta,kappa-caroten-6-one |
| Molecular formula | C40H56O3 |
| SMILES | O=C([C@@]2(C)C(C)(C)C[C@H](O)C2)/C=C/C(C)=C/C=C/C(C)=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1=[C@@](C)C[C@@H](O)CC(C)1C |
| Molecular weight | 584.9gmol-1 |
| Appearance | Red oil (Dark) |
| CAS number | [465-42-9] |
| Merck Index | [13:1775] |
| Einecs number | [207-364-1] |
| Solubility (in water) | soluble |
| Stability | Stable in ordinary conditions2 |
Antioxidant Power
- Definition: The ability to limit the damage caused by free radicals (damage due to oxygen) Simply put, oxygen radicals are soluble in the body, and in particular like to attack DNA. Therefore using something like vitamen C, or in this case Capsanthin, these radicals can be 'scavanged'. The radical scavanging ability of Capsanthin is particularly good, and noted to be better than beta carotene and lutein. The number of conjugated double bonds, functional groups and chain structure are what control the antioxidant effects. It is believed that the antioxidant power of Capsanthin is enhanced by the conjugated keto groups present in its structure. Capsanthin was found to decompose more slowly than other carotenoids, indicating that its antioxidant effects may last longer than other carotenoids, and hence it is better than beta-carotene and lutein. That is to say it has a more effective radical scavanging ability. In addition, it was found that esterified Capsanthin was also a good antioxidant based on analysis of this radical scavanging ability3
Synthesis of Capsanthin
The synthesis of Capsanthin has been studied by Yamano and Ito from Kobe pharmaceutical University. Their method involves the Regio and stereoselective rearrangement of an epoxide that is tetrasubstituted by utilising a lewis acid. For the full journal concerning this synthesis, the following link can be pursued: http://www.jstage.jst.go.jp/article/cpb/49/12/1662/_pdf
Also, a particular xanthophyll found in green unripe peppers and many vegetables is violaxanthin. This contains an epoxide ring, and the properties associated with the epoxide allow contraction of the six-membered ring on violaxanthin to form the ring (5-membered) found in Capsanthin. Thus violaxanthin is a natural precursor of capsanthin.1 This type of reaction occurs during the ripening process of green peppers.
Structure of violaxanthin
Analysis of Capsanthin
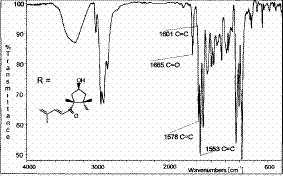
IR spectrum
In the IR spectra above, only the end group that differentiates Capsanthin from other carotenoids is analysed. Quite clearly, the O-H is shown by the broad stretch at approximately 3300cm-1. The other characteristic peaks, notably C=O and C=C are shown on the spetra.
Nmr spectrum
Barber, Jackman, Warren and Weddon note that the nmr of Capsanthin contains methyl bands at 9.02, 8.95, 8.77, 8.65, 8.3 and 8.02ppm in the ratios of 1:2:1:1:1:4. It was deduced that the methyl groups present on the cyclohexane ring give rise to the bands at 8.95 and 8.3 as they are also observed in zeaxanthin, which also contains the cyclohexane ring. 8.02ppm refers to the four methyl groups present in the Capsanthin chain. The remaining bands at 9.02, 8.77 and 8.65 show evidence for non-allylic methyl groups on the cyclopentane ring. This spectrum is noted to be very similar to capsanthone and capsorubin with respect to the cyclopentane ring hydrogens.
References
3. http://pubs.acs.org/cgi-bin/abstract.cgi/jafcau/1998/46/i09/abs/jf980200i.html
4. http://www3.interscience.wiley.com/cgi-bin/fulltext/95015751/PDFSTART