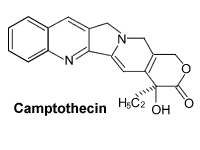
It:Camptothecin
| Template:Chemical header| structure of camptothecin |
|---|
 |
| Template:Chemical header| Chemical Data | |
|---|---|
| IUPAC name | |
| Appearance | Light Yellow Crystal |
| Molecular formula | C20H16N2O4 |
| Molecular mass | 348.36g/mol |
| Solubility | Clear Yellow Solution at 1Mg/Ml in Dimethylsulfoxide |
| PubMed ID | [ |
| PubChem ID | [] |
| Melting point | 260-240°C |
| Boiling point | ..... |
| CAS number | |
| smiles string | CC[C@@]1(O)C(=O)OCc2c(=O) n3c(Cc4nc5ccccc5cc34)cc12 |
Description
Camptothecin is an alkaloid derived from the Chinese "happy tree",Camptotheca acuminata Decne. It is one of the two most important compounds in cancer chemotherapy,which the other one is Taxol. Presently, first-generation analogs of camptothecin, such as Hycamtin (topotecan) and Camptosar (irinotecan or CPT-11), are used for the treatment of ovarian and colon cancer.
3D Image of Camptothecin
The origin of Camptothecin

The discovery of Camptothecin
In 1966, Drs. Wall and Wani and their colleagues reported the discovery of a compound they termed camptothecin from the Chinese tree Camptotheca acuminata. Nearly 20 years later, the unique mode of action for this potent cytotoxic compound was found to be the inhibition of an enzyme known as DNA Topoisomerase I. Camptothecin traps this enzyme, inhibiting DNA replication and killing the cancer cells.
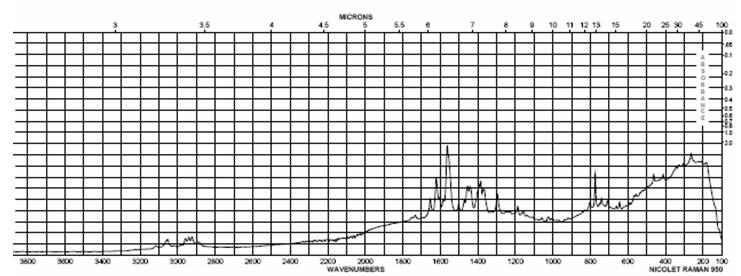
The IR spectrum of Camptothecin
Bibliographic Information
Sequence dependent modulating effect of camptothecin on the DNA-cleaving activity of the calf thymus type I topoisomerase.
High-resolution mapping of DNA topoisomerase I (I) cleavages in the regions of human DNA, including the oncogene c-Ha-ras and p53, revealed three kinds of I cleavage sites: 1.cleavage sites not affected by camptothecin; 2.cleavage sites reinforced only in the presence of camptothecin, and 3.cleavage sites which weaken in the presence of camptothecin. The preferences in camptothecin-reduced sites predominated upstream from the cleavage point, whereas in camptothecin-induced sites the situation was reversed.
External links
- Camptothecin Project From the Stehlin Foundation for Cancer Research
A novel solvent pH change solubilization method
